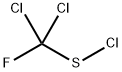
DICHLOROFLUOROMETHANESULFENYL CHLORIDE synthesis
- Product Name:DICHLOROFLUOROMETHANESULFENYL CHLORIDE
- CAS Number:2712-93-8
- Molecular formula:CCl3FS
- Molecular Weight:169.43
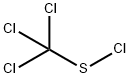
594-42-3
0 suppliers
$95.00/25g

2712-93-8
10 suppliers
inquiry
Yield: 66.4%
Reaction Conditions:
with iron(III) trifluoride;18-crown-6 ether;pyridine hydrogenfluoride in toluene at 103 - 108;Temperature;
Steps:
10 Fluorodichloromethylsulfonyl chloride
A. Perchloromethyl mercaptan, Iron fluoride Iron fluoride, Pyridine hydrofluoride solution and 18-crown ether were sequentially added to toluene, Heating to 95-125 ° C, reacting for 8-10 hours; B. The reaction solution was cooled to room temperature and filtered to remove insoluble matter. C. Atmospheric distillation to obtain a toluene solution of fluorodichloromethylsulfonyl chloride, Fractions of 92 to 96 ° C were collected. The mole ratio of perchloromethyl mercaptan to ferric fluoride was 1: 0.8; The mass ratio of perchloromethyl mercaptan and pyridine hydrofluoride solution was 1: 0.02. The mass ratio of 18-crown_6 and 18-crown-ether was 1: 0. 02; The mass ratio of total chloromethyl mercaptan to toluene was 1: 4. The yield of fluorodichloromethylsulfonyl chloride in this example was 64.8%.Similar to Example 8, except that the reaction temperature was 103 to 108 ° C. The yield of fluorodichloromethylsulfonyl chloride in this example was 66.4%.
References:
AstaTech (Chengdu) Biopharmaceutical Co., Ltd.;Huang, Yongxue;Kang, Xinglong;Wang, Yin;Xie, Guobin;He, Yan;Guo, Peng CN103755600, 2016, B Location in patent:Paragraph 0079-0082; 0084; 0087; 0088
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

2712-93-8
10 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

2712-93-8
10 suppliers
inquiry
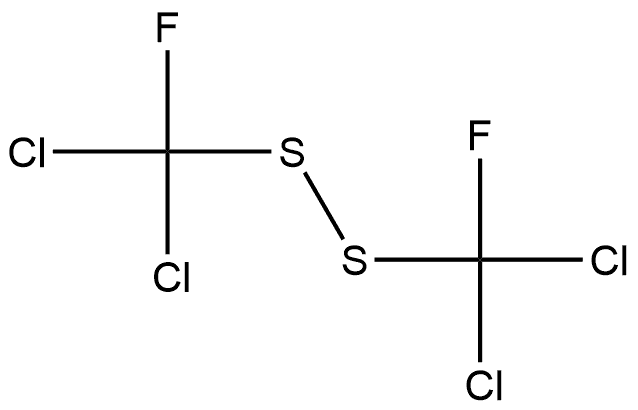
675-63-8
0 suppliers
inquiry

2712-93-8
10 suppliers
inquiry
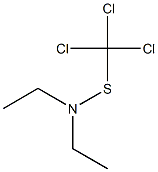
13029-20-4
0 suppliers
inquiry

2712-93-8
10 suppliers
inquiry