
(DIETHOXYPHOSPHORYL)DIFLUOROMETHYLZINC BROMIDE synthesis
- Product Name:(DIETHOXYPHOSPHORYL)DIFLUOROMETHYLZINC BROMIDE
- CAS Number:82845-20-3
- Molecular formula:C5H10BrF2O3PZn
- Molecular Weight:332.39
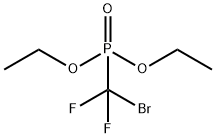
65094-22-6
213 suppliers
$6.00/1g

82845-20-3
9 suppliers
inquiry
Yield:-
Reaction Conditions:
with zinc in N,N-dimethyl acetamide at 20 - 45; for 3 h;
Steps:
2 EXAMPLE 2; Synthesis of 3-{4-[(Diethoxy-phosphoryl)-difluoro-methyl]-phenyl}-acrylic acid methyl ester (2)
To a stirred suspension of activated zinc dust (70 mmol) in 35 mL dry dimethylacetamide (DMA) is added slowly a solution of diethyl(bromodifluoromethyl)phosphonate (70 mmol) in 35 mL DMA under a nitrogen atmosphere. The reaction mixture is stirred for 1 hour at room temperature, heated to 45° C. for 1 hour and then room temperature for a further hour. Copper bromide (70 mmol) is added and the resulting mixture is stirred for 45 minutes. A suspension of methyl 4-iodocinnamate 1 (35 mmol) in 30 mL DMA is poured into the reaction mixture. The resulting reaction mixture is subjected to sonication for 24 hours under a nitrogen atmosphere. After this period of time, thin layer chromatography indicates that all the starting material has been consumed. The reaction mixture is then partitioned between water and ether. The biphasic mixture is passed through celite and extracted with ether. The organic extracts are washed with brine and dried over sodium sulfate. The volatiles are removed in vacuo, and the residue is chromatographed on silica gel (hexane:ethyl acetate 50:3) to yield the desired cinnamate ester 2 as a light colored solid (75%). 1H NMR (CDCl3): 7.67(d, J=16Hz, 1H), 7.61(m, 4H), 6.47(d, J=16Hz, 1H), 4.25(m, 4H), 3.79(s, 3H), 1.32(m, 6H).
References:
US2004/225146,2004,A1 Location in patent:Page 3

65094-22-6
213 suppliers
$6.00/1g
1478-53-1
93 suppliers
$16.00/500mg

82845-20-3
9 suppliers
inquiry