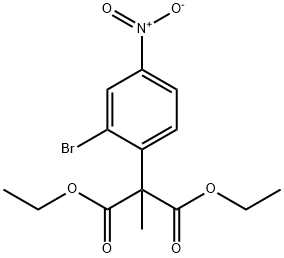
Diethyl 2-(2-bromo-4-nitrophenyl)-2-methylmalonate synthesis
- Product Name:Diethyl 2-(2-bromo-4-nitrophenyl)-2-methylmalonate
- CAS Number:945244-26-8
- Molecular formula:C14H16BrNO6
- Molecular Weight:374.18
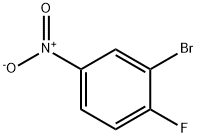
701-45-1
280 suppliers
$9.00/1g

945244-26-8
30 suppliers
inquiry
Yield:945244-26-8 99%
Reaction Conditions:
Stage #1: Diethyl methylmalonatewith sodium hydride in N,N-dimethyl-formamide at 0 - 20; for 0.216667 h;
Stage #2: 2-bromo-1-fluoro-4-nitrobenzene in N,N-dimethyl-formamide at 20; for 0.166667 h;
Steps:
42.a
Step a: Diethyl 2-(2-bromo-4-nitrophenyl)-2-methylmalonate Diethyl 2-methylmalonate (4.31 mL, 25.0 mmol) was dissolved in 25 mL of anhydrous DMF. This solution was cooled to 0° C. under an atmosphere of nitrogen. Sodium hydride (1.04 g, 26 mmol, 60% by weight in mineral oil) was slowly added to the solution. The resulting mixture was allowed to stir for 3 minutes at 0° C., and then at room temperature for 10 minutes. 2-Bromo-1-fluoro-4-nitrobenzene (5.00 g, 22.7 mmol) was quickly added and the mixture turned bright red. After stirring for 10 minutes at room temperature, the crude mixture was evaporated to dryness and then partitioned between dichloromethane and a saturated aqueous solution of sodium chloride. The layers were separated and the organic phase was washed twice with a saturated aqueous solution of sodium chloride. The organics were concentrated to yield diethyl 2-(2-bromo-4-nitrophenyl)-2-methylmalonate (8.4 g, 99%) as a pale yellow oil which was used without further purification. Retention time 1.86 min.
References:
US2008/9524,2008,A1 Location in patent:Page/Page column 406
