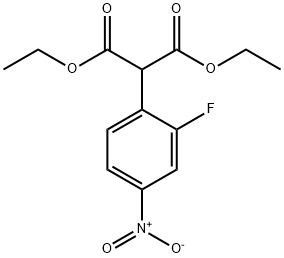
Diethyl 2-(2-fluoro-4-nitrophenyl)Malonate synthesis
- Product Name:Diethyl 2-(2-fluoro-4-nitrophenyl)Malonate
- CAS Number:318471-58-8
- Molecular formula:C13H14FNO6
- Molecular Weight:299.25

369-34-6
413 suppliers
$5.00/5g
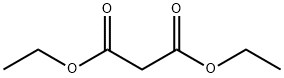
105-53-3
778 suppliers
$5.00/25g

318471-58-8
11 suppliers
$55.00/250mg
Yield:318471-58-8 100%
Reaction Conditions:
Stage #1: diethyl malonatewith sodium hydride in N,N-dimethyl-formamide at 0; for 0.166667 h;
Stage #2: 3,4-difluoronitrobenzene in N,N-dimethyl-formamide at 70;
Steps:
26.A
Step A: Diethyl (2-fluoro-4-nitrophenyl)propanedioate; To a suspension of 60% NaH (5.28 g, 131.99 mmol) in dry 100 ml_ of DMF, diethyl malonate (20.11 g, 125.71 mmol) was added dropwise while cooling to O0C. After stirring for 10 min at O0C, a solution of 3,4-difluoronitrobenzene (10.00 g, 62.85 mmol) in 10 ml_ of DMF was added dropwise. The reaction was stirred overnight while heating to 700C and then cooled to RT, quenched with sat. aqueous NH4CI, and extracted with EtOAc x4. Combined organics were washed with brine x3 and dried over MgSO4. Silica gel was added and the solvent was removed under
References:
WO2009/76140,2009,A1 Location in patent:Page/Page column 133-134