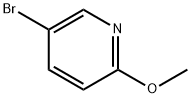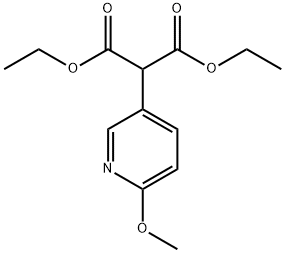
Diethyl 2-(6-Methoxy-3-pyridyl)Malonate synthesis
- Product Name:Diethyl 2-(6-Methoxy-3-pyridyl)Malonate
- CAS Number:902130-84-1
- Molecular formula:C13H17NO5
- Molecular Weight:267.28
Yield:902130-84-1 78%
Reaction Conditions:
with benzoxazole;copper(l) iodide;triethylamine in dimethyl sulfoxide at 60; for 0.25 h;
Steps:
9.1; 9.2; 9.3; 9.4 Example 9:
(1) 2-Methoxy-5-bromopyridine was dissolved in DMSO at a concentration of 1 mol / L to give solution a; (2) diethyl malonate, benzoxazole, triethylamine The molar ratio of diethyl malonate to 2-methoxy-5-bromopyridine was 1.5: 1 and the molar ratio of benzoxazole to 2-methoxy-5-bromopyridine was 0.4 : 1, the molar ratio of triethylamine to 2-methoxy-5-bromopyridine is 4: 1 to obtain solution b; (3) adding solid catalyst cuprous iodide to a fixed bed microchannel reaction apparatus, 2-methoxy-5-bromopyridine was 0.2: 1; (4) the solution a and the solution b were simultaneously pumped into the fixed bed microchannel reaction apparatus after the treatment in step (3) The microreactor in the device (diameter 0.5 mm, length 20 m) reacts.The flow rate ratio of solution a and solution b is controlled at 1: 1, flow rate 1ml / min, holding residence time 15min, reaction at 60 ; (5) collecting outflow liquid, EA / water extraction, combining organic phase, adding anhydrous sulfuric acid The product was purified by silica gel column chromatography and separated by petroleum ether / ethyl acetate as mobile phase. The product was pure product. The yield was 78%.
References:
CN106674091,2017,A Location in patent:Paragraph 0046; 0047
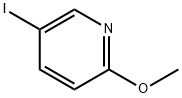
13472-61-2
81 suppliers
$6.00/250mg
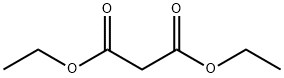
105-53-3
733 suppliers
$5.00/25g

902130-84-1
12 suppliers
inquiry