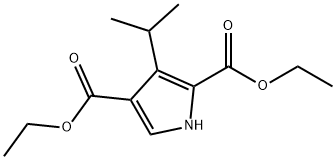
DIETHYL 3-ISOPROPYL-1H-PYRROLE-2,4-DICARBOXYLATE synthesis
- Product Name:DIETHYL 3-ISOPROPYL-1H-PYRROLE-2,4-DICARBOXYLATE
- CAS Number:651744-38-6
- Molecular formula:C13H19NO4
- Molecular Weight:253.29
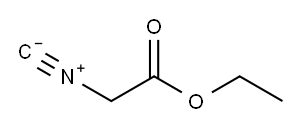
2999-46-4
220 suppliers
$13.43/1gm:
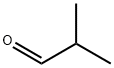
78-84-2
362 suppliers
$10.00/25mL

651744-38-6
30 suppliers
$300.00/100mg
Yield:651744-38-6 60%
Reaction Conditions:
with 1,8-diazabicyclo[5.4.0]undec-7-ene in tetrahydrofuran at 20; for 19 h;
Steps:
1.A Example 1; [5- (1-METHYLETHYL) PYRROLO] [2, [1-F]] [1, 2,4] triazin-4 [(3H)-ONE-6-CARBOXYLIC] acid ethyl ester
A) Ethyl isocyanoacetate (80 g, 0.71 mol) was dissolved in 1 L of dry tetrahydrofuran under nitrogen and 1, 8-diazabicyclo [5.4. [0] UNDEC-7-ENE] (107.7g, 0.71 mol) was added to the solution. A solution of isobutyraldehyde (29.7g, 0.41 moles) in 1.5 L of dry tetrahydrofuran was added dropwise at room temperature over 3 hours. The mixture was then stirred at room temperature for 16 h. The reaction mixture was concentrated under vacuum to a brown oil. The concentrate was partitioned between 1.2 L of ethyl, acetate and 0.5 L of water. The organic layer was then washed with 0.4 L of 0.1 N hydrochloric acid followed by 0.3 L of saturated sodium bicarbonate solution and then 0.3 L of saturated brine. The organic layer was dried (sodium sulfate), filtered and concentrated under vacuum to a brown oil. The residue was dissolved in toluene and added to a 1600 ml [(-800] g) column of silica gel wet with hexane. Product was eluted at 15 PSI nitrogen pressure first with 4.8 L of hexane followed by 5 L of 20% ethyl acetate in hexane. Eluent containing product by TLC analysis was combined and concentrated under vacuum to a yellow oil. The concentrate was pumped dry under high vacuum giving product A, [3- (1-] methylethyl) pyrrole-2,4-dicarboxylic acid diethyl ester (54g, 60% yield) of yellow oil that solidified on standing at room temperature. TLC silica gel: [RF =] 0.2, hexane/ethyl acetate (4/1) uv visualization and PMA stain. 1H NMR [(CDC13)] 8 1.2-1. 5 (m, 12H), 4.2-4. 3 (m, 1H), 4.3-4. 3 (m, 4H), 7.5 (d, 1H).
References:
WO2004/13145,2004,A1 Location in patent:Page 29