
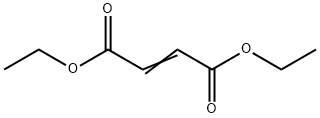
diethyl cyclohex-4-ene-1,2-dicarboxylate synthesis
- Product Name:diethyl cyclohex-4-ene-1,2-dicarboxylate
- CAS Number:13043-60-2
- Molecular formula:C12H18O4
- Molecular Weight:226.2689
Yield:13043-60-2 89 %
Reaction Conditions:
with sulfuric acidReflux;
Steps:
4.1 Step 1: Preparation of compound III (diethyl cyclohex-4-ene-1,2-dicarboxylate)
Add 300g of tetrahydrophthalic anhydride powder into a 2L four-neck round bottom flask, add 700g of ethanol, start stirring, then slowly drop in 14.5g of concentrated sulfuric acid, start timing when heating to reflux, and distill out ethanol after half an hour of reflux, every half hour GC analysis, and add an equal amount of ethanol according to the amount of distilled ethanol.Reflux for 3 hours and the reaction is over, the selectivity is above 96%, the reaction is completely cooled to room temperature, 16.5 g of sodium carbonate is added, the unreacted sulfuric acid is removed by stirring, the solid in the reaction system is removed by suction filtration, the ethanol is spin-dried to obtain the crude product, and the crude product is After vacuum distillation, 401 g of compound III with a purity of more than 99% was obtained (89% molar yield)
References:
CN115232058,2022,A Location in patent:Paragraph 0147-0150
1520-50-9
0 suppliers
inquiry

13043-60-2
0 suppliers
inquiry

