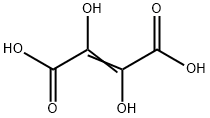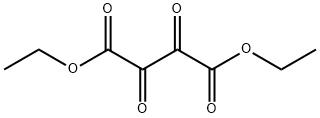
Diethyldioxosuccinate synthesis
- Product Name:Diethyldioxosuccinate
- CAS Number:59743-08-7
- Molecular formula:C8H10O6
- Molecular Weight:202.16
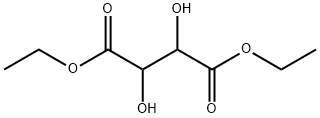
408332-88-7
2 suppliers
inquiry

59743-08-7
4 suppliers
inquiry
Yield:-
Reaction Conditions:
with N-Bromosuccinimide in 1,2-dichloro-ethane; for 2 h;Reflux;Inert atmosphere;
Steps:
4.2. Glycoluril diethyl ester (1)
A mixture of diethyl tartrate (15.0 g, 72.8 mmol) and NBS (17.0 g,218 mmol) in dry ethylene dichloride (EDC, 100 mL) was heatedunder reflux for 2 h under N2 atmosphere. When the reaction wascompleted, Na2SO3 (10 g) was added to quench the residual bromine.The reaction mixture was evaporated to remove EDC. Thenthe residuewas diluted with diethyl ether (40 mL), filtered, and thesolid washed with ether (40 mL4) to remove succinimide and thesalts. To the combined filtrates was added urea (10.9 g, 102 mmol)and the resulting solution was evaporated under reduced pressure.To the yellow oil residue was added toluene (50 mL) and the solutionwas evaporated again to remove residual H2O. To theresulting mixture, toluene (150 mL) and TFA (16.5 mL, 286 mmol)were added. The reaction flask was equipped with a DeaneStarktrap and heated under reflux. The initial distillate (ca. 50 mL) wasdrained off and the reaction mixture was heated for another 6e8 h.Then, the reaction mixture was cooled to room temperature andthe solvent was removed under reduced pressure. EtOH (100 mL)was added to the residue and the mixture was stirred overnight atroom temperature. The precipitate was collected by filtration followedby washing with acetone (150 mL) and water (10 mL). Thecollected solid was dried in air to give 1 as a white solid
References:
Shin, Moonyong;Kim, Myeong Hak;Ha, Tae-Hwan;Jeon, Jongho;Chung, Kyoo-Hyun;Kim, Jin Seuk;Kim, Young Gyu [Tetrahedron,2014,vol. 70,# 8,p. 1617 - 1620]
87-91-2
444 suppliers
$17.46/25G

59743-08-7
4 suppliers
inquiry