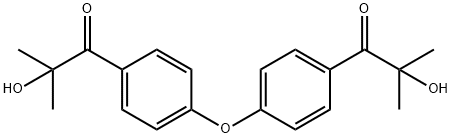
Esacure KIP 160 synthesis
- Product Name:Esacure KIP 160
- CAS Number:71868-15-0
- Molecular formula:C20H22O5
- Molecular Weight:342.39
157891-77-5
10 suppliers
$559.00/1g

71868-15-0
37 suppliers
inquiry
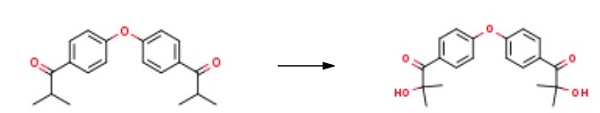
Yield: 90%
Reaction Conditions:
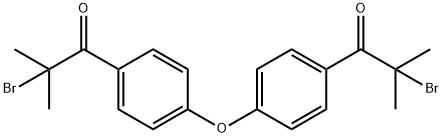
Stage #1:bis<4-(2-bromisobutyryl)phenyl> ether with sodium disulfite in water at 85; for 0.166667 h;
Stage #2: with sodium hydroxide in water;isopropyl alcohol for 0.25 h;Product distribution / selectivity;Reflux;
Steps:
3.c
The suspension of the di-bromo intermediate obtained according to Method F or G was stirred with 3g of a Na2S2θs 10% water solution at 85°Cfor 10', then diluted with 10.6g of i-propyl alcohol and 2.6g of water. To the so obtained solution 2.3g of
References:
LAMBERTI SPA WO2009/135895, 2009, A1 Location in patent:Page/Page column 16-17
157891-84-4
7 suppliers
$653.00/1g

71868-15-0
37 suppliers
inquiry

649757-85-7
0 suppliers
inquiry

71868-15-0
37 suppliers
inquiry
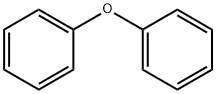
101-84-8
584 suppliers
$5.00/25g

71868-15-0
37 suppliers
inquiry
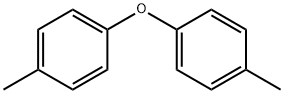
1579-40-4
167 suppliers
$31.89/5g

71868-15-0
37 suppliers
inquiry