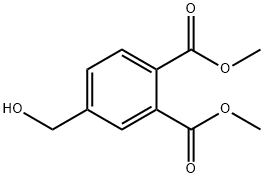
dimethyl 4-(hydroxymethyl)benzene-1,2-dioate synthesis
- Product Name:dimethyl 4-(hydroxymethyl)benzene-1,2-dioate
- CAS Number:170433-63-3
- Molecular formula:C11H12O5
- Molecular Weight:224.21
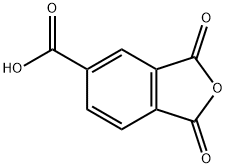
552-30-7
400 suppliers
$16.00/25g

170433-63-3
1 suppliers
inquiry
Yield:-
Reaction Conditions:
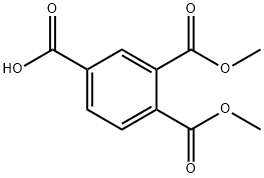
Stage #1: trimellitic Anhydridewith borane-THF in 1,4-dioxane at 20; for 13.5 h;
Stage #2: with ammonium chloride in 1,4-dioxane;dichloromethane;water;
Stage #3: with sulfuric acid in methanol;water; for 18 h;Heating / reflux;
Steps:
1.a EXAMPLE 1; {5-[4'(1-Ethyl-1-hydroxypropyl)biphenyl-3-yloxymethyl]-2-hydroxymethylphenyl}methanol, a) Dimethyl 4-hydroxymethylphthalate
1,2,4-benzenetricarboxylic anhydride (50 g, 260 mmol) is dissolved in 800 mL of anhydrous dioxane. BH3.THF (260 mmol, 1 eq.) is added dropwise via a dropping funnel, over a period of about 1 h 30 min at room temperature. Stirring is maintained for 12 h and the reaction medium is then poured into a mixture containing 600 mL of saturated ammonium chloride solution and 2 L of dichloromethane. After separation of the phases by settling, the organic phase is dried and the solvents are evaporated off under reduced pressure. The residue obtained is then dissolved in 1 L of methanol and heated to reflux, after addition of 5 mL of sulphuric acid. After reflux for 18 h, the reaction medium is cooled to room temperature and poured directly into a water/ethyl ether mixture (1 L/2 L). After separation of the phases by settling, the aqueous phase is re-extracted with two fractions of ethyl ether (about 700 mL) and the organic phases are then combined, dried and concentrated under reduced pressure. A triester-diester/alcohol mixture is obtained in a yield of 80%, containing 65% of the desired product.
References:
US6831106,2004,B1 Location in patent:Page column 12
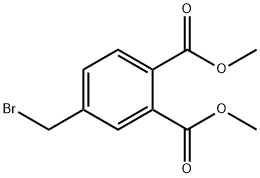
20116-67-0
0 suppliers
inquiry

170433-63-3
1 suppliers
inquiry