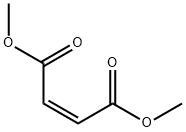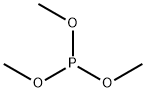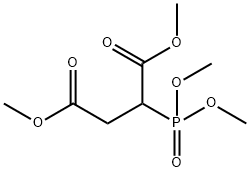
dimethyl (dimethoxyphosphinyl)succinate synthesis
- Product Name:dimethyl (dimethoxyphosphinyl)succinate
- CAS Number:2788-26-3
- Molecular formula:C8H15O7P
- Molecular Weight:254.17
Yield:2788-26-3 92.4%
Reaction Conditions:
with formic acid at 90 - 100; for 11.5 h;
Steps:
3 Example 3:
Add dimethyl maleate 288.3g to a four-necked flask with stirring, thermometer and constant pressure dropping funnel(2mol) and 27g (0.6mol) of formic acid were heated to 90 ° C, and 297.8 g (2.4 mol) of trimethyl phosphinate was added dropwise, and the mixture was added dropwise.The speed control reaction temperature is about 100 ° C, and the addition is completed in about 1.5 h. Insulation reaction for 10h, the reaction is(GC monitoring reaction endPoint), the reaction liquid is poured into a rotary steaming bottle and rotary evaporated by a water pump, and the methyl formate formed in the system is first unscrewed to raise the oil temperature.After rising to 130 ° C, the remaining low boiling impurities were distilled off to obtain 565.5 g of the compound of the formula VI, and the compound of the formula VI wasReddish brown oil, GC content 95%, yield 92.4%;382.5 g (1.5 mol) of the compound of the formula VI structure was added to a three-necked flask, and 300 g of water was added thereto, and the system was white turbid, and the internal temperature was cooled to about 6 ° C, and 10 mol / was added dropwise under stirring.L sodium hydroxide solution 315ml (3.15mol), drip acceleration control internal temperature does not exceed 20 ° C, about 10min after the completion of the addition, the system becomes clear, continuous stirring at 16 ° C for 2h, TLC analysis, the raw material is completely reacted, the internal temperature 312g (3.15mol) concentrated hydrochloric acid was added dropwise at 16 °C, and the addition was completed in about 10 minutes. At this time, the internal temperature was raised to 25 ° C. After stirring for 30 min, 200 g of chlorine was added.The aqueous phase was saturated with sodium chloride, and then extracted with dichloromethane (250 ml×2). The organic phase was combined, driedanhydrous magnesium sulfate, filtered, and evaporated to give a compound of the compound of formula VIII (325.8 g). Yellow transparent oil, HPLC, analytical purity 95.6%, yield 95.7%;A compound of the formula VIII was added to a three-necked flask, 272.4 g (1.2 mol).With acetic anhydride 367.5g (3.6mol), heated to an internal temperature of 40 ° C, the heating oil bath was removed, the reaction began to heat up, the temperature inside the bottle climbed from 40 ° C to 75 ° C and began to fall, reset in the oil bath In the middle, the internal temperature was maintained at 75 ° C for 1 h and then observed by TLC, the starting material was completely reacted. Adding a distillation unit at an internal temperature of 75 ° C, first using a water pump to distill off excess acetic anhydride and the produced acetic acid, noAfter distillate, the oil pump was used to continue distillation. Finally, the internal temperature was raised to 95 ° C, and trace amounts of acetic anhydride and acetic acid in the system were drained to obtain 229.5 g of the phosphine-based flame retardant monomer of the formula I. The rate of 91.5%, the phosphine-based reactive flame retardant monomer of the formula I structure is a product of a yellowish oil.
References:
CN109336927,2019,A Location in patent:Paragraph 0060; 0061