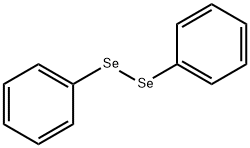
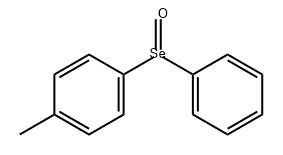
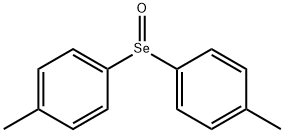
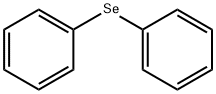
Diphenyl selenoxide synthesis
- Product Name:Diphenyl selenoxide
- CAS Number:7304-91-8
- Molecular formula:C12H10OSe
- Molecular Weight:249.17
1132-39-4
55 suppliers
$31.28/1g

7304-91-8
3 suppliers
inquiry
Yield:7304-91-8 94%
Reaction Conditions:
with N-chloro-succinimide in methanol;dichloromethane at 0; for 0.5 h;
Steps:
4.a a) Synthesis of Seleninyldibenzene III
General procedure: GP 2: In a 50 ml two-neck flask, 1.0 eq. of the organodiphenyl selenide II compound was dissolved in a 1:1 mixture of dichloromethane/methanol (13.4 ml/1.0 mmol) and cooled to 0° C. Subsequently, 1.05 eq. of N-chlorosuccinimide were added and the mixture was stirred at 0° C. for 30 minutes, in the course of which a pale yellow color of the solution was observed. Subsequently, a saturated NaHCO3 solution (1.0 ml/1.0 mmol) was added, the mixture was stirred for 15 minutes, water (15 ml/1.0 mmol) was added and the mixture was stirred once again at 0° C. for 15 minutes. And the organic phase was washed with water (3×25 ml/1.0 mmol). The aqueous phase was extracted with dichloromethane (3×25 ml/1.0 mmol) and driedmagnesium sulphate. The desiccant was filtered off, the solvent was removed under reduced pressure and the crude product was dried under vacuum at 50° C. for three hours. According to GP 2, 175 μl (234 mg, 1.00 mmol, 1.0 eq) of diphenyl selenide were reacted with 140 mg (1.05 mmol, 1.05 eq) of N-chlorosuccinimide.
After extractive workup, 235 mg (0.940 mmol, 94%) of the title compound III were obtained as a colourless solid.
IR (ATR): {circumflex(υ)} (cm-1)=3044; 3008; 2989; 2941; 1570; 1470; 1437; 1300; 1156; 1069; 1056; 1047; 1017; 993; 915; 850; 820; 733; 686; 611; 481; 442, 1H NMR (300 MHz, toluene-d8): δ (ppm)=7.67-7.51 (m, 4H, Ar-CH); 7.16-6.87 (m, 6H, Ar-CH); 13C NMR (75 MHz, toluene-d8): δ (ppm)=145.1; 130.5; 129.3; 126.0; 77Se NMR (57 MHz, toluene-d8): δ (ppm)=851.0; HR-MS (ESI-TOF): calc. for C12H11OSe ([M+H]+): 250.99700, found: 250.99691; calc. for C12H10OSeNa ([M+Na]+): 272.97894, found: 272.97888; C12H10OSe (249.99 g/mol).
The analytical data are in agreement with the literature data.
References:
US2017/158726,2017,A1 Location in patent:Paragraph 0129-0132
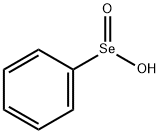
6996-92-5
124 suppliers
$14.00/250mg

98-80-6
718 suppliers
$5.00/5g

1132-39-4
55 suppliers
$31.28/1g

7304-91-8
3 suppliers
inquiry