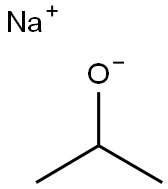
Dodecyl isopropyl ether synthesis
- Product Name:Dodecyl isopropyl ether
- CAS Number:29379-42-8
- Molecular formula:C15H32O
- Molecular Weight:0
Yield:29379-42-8 120.3 g
Reaction Conditions:
Stage #1: isopropanolwith natrium at 60 - 90; for 1 h;
Stage #2: 1-dodecylbromidewith potassium iodide at 60 - 80; for 12 h;
Steps:
16 Synthesis of n-dodecyl-isopropyl ether
In a 500ml three-necked bottle, equipped with refluxing condenser, thermostat, thermometer, magnetic stirrer, dropping funnel and gas outlet tube 208 g (3.48 mol) iso-propyl alcohol were placed and heated to 60°C. 7.29g (0.32mol) sodium was added in thin slices over 15 minutes. The temperature increased to 90°C during the addition, and the reaction mixture was maintained at this temperature until the sodium was dissolved (1h). The mixture was cooled to 60°C. Then, 59.8 g (0.12mol) 1 -bromododecane and 0.05g KI were added, and the mixture was heated to 80°C for 12 h. After a few minutes a solid started to precipitate. (0480) The solid was filtered off and the liquid phase was mixed with 1400ml DI water, 500 g NaCI and 100 ml n-hexane. The upper organic layer was separated, and the aqueous layer was extracted once more with 100 ml n-hexane. The organic layers were combined (500 ml) and dried over NaCI. This reaction sequence was repeated two times in identical scale (in total three batches). (0481) Distillation of the combined organic phases of the three batches under reduced pressure yielded 120.3 g n-Dodecyl-isopropyl ether (b.p. 149-151 °C at 9 mbar; purity of 99.5 % as determined by GC analysis).
References:
WO2022/152742,2022,A1 Location in patent:Page/Page column 125

84988-08-9
0 suppliers
inquiry
67-63-0
1556 suppliers
$17.00/25ML

112-41-4
218 suppliers
$8.00/10g

29379-42-8
0 suppliers
inquiry

112-53-8
430 suppliers
$10.00/5g

112-53-8
430 suppliers
$10.00/5g

29379-42-8
0 suppliers
inquiry
![Dodecane, 1-[(1-methylethenyl)oxy]-](/CAS/20210305/GIF/52169-28-5.gif)
52169-28-5
0 suppliers
inquiry

29379-42-8
0 suppliers
inquiry