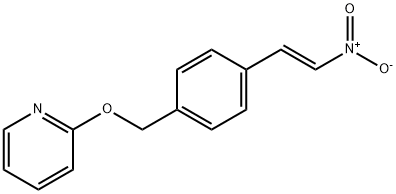
(E)-2-((4-(2-nitrovinyl)benzyl)oxy)pyridine synthesis
- Product Name:(E)-2-((4-(2-nitrovinyl)benzyl)oxy)pyridine
- CAS Number:936342-26-6
- Molecular formula:C14H12N2O3
- Molecular Weight:256.26
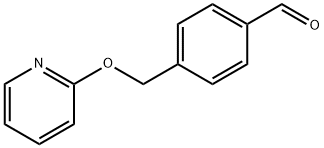
936342-25-5
19 suppliers
$1000.00/5G

75-52-5
4 suppliers
$35.28/100g

936342-26-6
10 suppliers
inquiry
Yield:936342-26-6 74.5%
Reaction Conditions:
with ammonium acetate;acetic acid at 100; for 1.5 - 1.75 h;Product distribution / selectivity;
Steps:
2.1.3; 2.2.3
Manufacturing Example 2-1-3 2-(4-((E)-2-Nitro-vinyl)-benzyloxy)-pyridine; A mixture of 4-(pyridin-2-yloxymethyl)-benzaldehyde (23.4 g, 110 mmol) described in Manufacturing Example 2-1-2, nitromethane (33.6 g, 550 mmol), ammonium acetate (17.0 g, 220 mmol) and acetic acid (200 mL) was stirred for 1 hour and 45 minutes at 100° C. The reaction solution was stirred on an ice bath while adding a small amount of water, and the precipitated solids were filtered to obtain the title compound (21.0 g, 74.5%).1H-NMR Spectrum (DMSO-d6) δ (ppm): 5.41 (2H, s), 6.91 (1H, dd, J=0.8, 8.4 Hz), 6.99-7.10 (1H, m), 7.53 (2H, d, J=8.0 Hz), 7.72-7.79 (1H, m), 7.86 (2H, d, J=8.0 Hz), 8.13 (1H, d, J=10Hz), 8.15-8.20 (1H, m), 8.23 (1H, d, J=10 Hz).; Manufacturing Example 2-2-3 2-(4-((E)-2-nitro-vinyl)-benzyloxy)-pyridine A mixture of the 4-(pyridin-2-yloxymethyl)-benzaldehyde (26.8 g, 126 mmol) described in Manufacturing Example 2-2-2, nitromethane (34 mL, 630 mmol), ammonium acetate (19 g, 252 mmol) and acetic acid (90 mL) was stirred at 100° C. for 90 minutes. The reaction solution was partitioned into ethyl acetate and water. The organic layer was isolated, washed with water (5 times) and saturated aqueous sodium bicarbonate (once), dried over anhydrous magnesium sulfate, and then filtered. The filtrate was concentrated under a reduced pressure to obtain the title compound (31 g) as a crude product.
References:
US2009/82403,2009,A1 Location in patent:Page/Page column 53-54