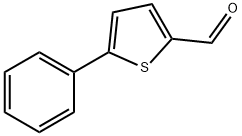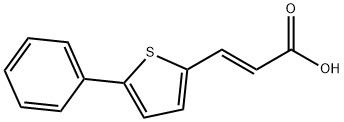
(E)-3-(5-phenylthiophen-2-yl)acrylic acid synthesis
- Product Name:(E)-3-(5-phenylthiophen-2-yl)acrylic acid
- CAS Number:58267-95-1
- Molecular formula:C13H10O2S
- Molecular Weight:230.28
Yield:58267-95-1 94%
Reaction Conditions:
with piperidine in pyridine; for 2 h;Reflux;Knoevenagel Condensation;
Steps:
2 General procedure for the synthesis of the 3-(5-bromo-2-thienyl)acrylic acid 4 and 3-(5-phenyl-2-thienyl)acrylic 5 via Knoevenagel condensation
General procedure: 2-Thiophenecarboxyaldehyde derivative (1 eq, 30 mmol) was dissolved in pyridine (60 mL) and piperidine (3 mL).
After addition of malonic acid (1.2 eq, 36 mmol), the mixture was refluxed for 2 h.
After cooling, the mixture was poured into a mixture of 200 mL water, 200 mL ice and 200 mL concentrated hydrochloric acid to yield a solid.
The precipitating solid was filtered off, washed with water and dried under pressure to give the corresponding 3-(2-thienyl)acrylic acid derivative.; 4.1.1.2
3-(5-phenyl-2-thienyl)acrylic acid 5
1H NMR (DMSO-d6, 250 MHz): δ = 12.16 (bs, 1H), 7.50-7.63 (m, 3H), 7.36-7.43 (m, 2H), 7.17-7.35 (m, 3H), 6.03 (d, J = 15.7 Hz, 1H); 13C NMR (DMSO-d6, 62 MHz): δ = 167.24, 146.11, 138.10, 136.66, 133.45, 132.90, 129.22, 128.50, 125.57, 124.89, 117.29; LC/MS (ESI): 228.96 [M-H]- and 231.10 [M+H]+; HRMS (FAB) cald for C13H11O2S (M + H)+ 231.0480, found 231.0486; purity > 99%; slight yellow powder.
Yield = 94%.
References:
Benmansour, Fatiha;Eydoux, Cécilia;Querat, Gilles;De Lamballerie, Xavier;Canard, Bruno;Alvarez, Karine;Guillemot, Jean-Claude;Barral, Karine [European Journal of Medicinal Chemistry,2016,vol. 109,p. 146 - 156]

4701-17-1
298 suppliers
$6.00/1g

58267-95-1
16 suppliers
inquiry

98-80-6
723 suppliers
$5.00/5g

58267-95-1
16 suppliers
inquiry