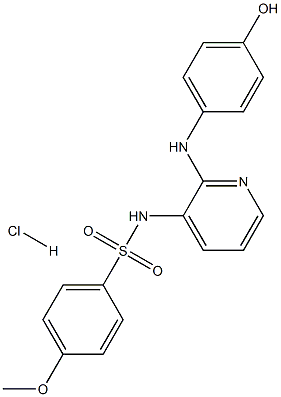
E-7010 monohydrochloride synthesis
- Product Name:E-7010 monohydrochloride
- CAS Number:141450-48-8
- Molecular formula:C18H18ClN3O4S
- Molecular Weight:407.8712
Yield:-
Reaction Conditions:
with pyridine in tetrahydrofuran at 0 - 25; for 0.25 h;
Steps:
3
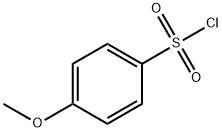
EXAMPLE 3; A mixture of EXAMPLE 2 in pyridine (9 mL/g) at 0° C. was treated with a mixture of para-methoxybenzenesulfonyl chloride (1.05 equivalents) in THF (1.4 mL/g) at 0° C. at a rate which kept the reaction temperature below 5° C., warmed to 25° C., stirred for 15 minutes, and concentrated. The concentrate was treated with n-propanol to provide a composition having 9% pyridine in the solvent mixture and to precipitate a solid. The mixture was cooled to 0° C. and filtered. The filtrant and washed with ethyl acetate (5-7 mL/g starting material) and dried at 45° C. This product may be milled until amorphous Amorphous N-(2-((4-hydroxyphenyl)amino)pyridin-3-yl)-4-methoxybenzenesulfonamide hydrochloride is characterized by a plain halo in its powder diffraction pattern, an absence of peaks characteristic of a crystalline N-(2-((4-hydroxyphenyl)amino)pyridin-3-yl)-4-methoxybenzenesulfonamide hydrochloride, or both.
References:
US2006/293368,2006,A1 Location in patent:Page/Page column 2
![4-[(3-AMINOPYRIDIN-2-YL)AMINO]PHENOL](/CAS/GIF/78750-68-2.gif)