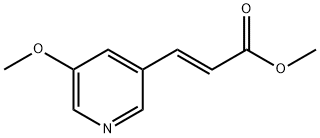
(E)-Methyl 3-(5-methoxypyridin-3-yl)acrylate synthesis
- Product Name:(E)-Methyl 3-(5-methoxypyridin-3-yl)acrylate
- CAS Number:1233560-96-7
- Molecular formula:C10H11NO3
- Molecular Weight:193.2
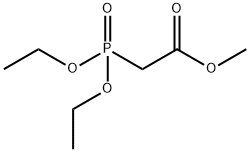
1067-74-9
212 suppliers
$5.00/5g
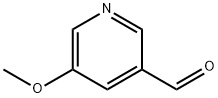
113118-83-5
142 suppliers
$13.00/250mg

1233560-96-7
10 suppliers
$45.00/10mg
Yield: 14%
Reaction Conditions:
Stage #1:Methyl diethylphosphonoacetate with sodium hydride in tetrahydrofuran;mineral oil at 0; for 0.5 h;Inert atmosphere;
Stage #2:5-methoxypyridine-3-carboxaldehyde in tetrahydrofuran;mineral oil at 0;
Steps:
21.1
21.1. methyl (2E)-3-(5-methoxypyridin-3-yl)prop-2-enoate To a suspension of 3.12 g (78.1 mmol) of sodium hydride (60% in oil) in 30 mL of anhydrous THF is added, over 45 minutes, under argon and at 0° C., 16.4 g (78.1 mmol) of methyl (diethoxyphosphoryl)acetate in 10 mL of THF. Stirring is maintained for 30 minutes at 0° C. and 5.1 g (37.2 mmol) of 5-methoxypyridine-3-carbaldehyde in 20 mL of anhydrous THF are then added dropwise at 0° C. After cooling to room temperature, the reaction mixture is treated with 150 mL of water and then extracted with EtOAc (3*100 mL). The organic phases are combined, washed successively with water (2*20 mL), dried over Na2SO4, filtered and then concentrated under reduced pressure. The residue obtained is purified by chromatography on a column of silica gel, eluting with a cyclohexane/EtOAC gradient of 0 to 40% EtOAc. 1 g of methyl (2E)-3-(5-methoxypyridin-3-yl)prop-2-enoate is obtained in the form of a white powder. Yield=14%. 1H NMR, CDCl3, 400 MHz, δ (ppm): 8.4 (d, 1H); 7.7 (d, 1H); 7.4 (s, 1H); 6.5 (d, 1H); 3.9 (s, 3H); 3.8 (s, 3H)
References:
SANOFI US2011/294788, 2011, A1 Location in patent:Page/Page column 25
50720-12-2
352 suppliers
$11.00/5g
292638-85-8
2 suppliers
inquiry

1233560-96-7
10 suppliers
$45.00/10mg