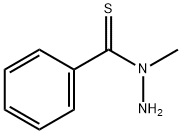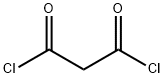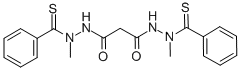
Elesclomol synthesis
- Product Name:Elesclomol
- CAS Number:488832-69-5
- Molecular formula:C19H20N4O2S2
- Molecular Weight:400.52
Yield: 82%
Reaction Conditions:
with N-(3-dimethylaminopropyl)-N-ethylcarbodiimide in N,N-dimethyl-formamide at 17 - 25; for 2 h;Product distribution / selectivity;Cooling with water-ice;
Steps:
2
Thiobenzoic acid N-methyl hydrazide (100 g, 1.0 eq) and malonic acid (35.1 g, 0.56 eq.) were dissolved in MN-dimethylformamide (DMF, 500 mL, 5 L/kg). 1- Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC, 150 g, 1.5 kg/kg, 1.3 eq.) was added in portion maintaining the internal temperature of the reaction mixture less than 22 0C using water-ice bath. After 2h at room temperature (17 ~ 25 0C) , the reaction was diluted with ethyl alcohol (SDA 3 A 200 proof, 200 mL, 5 L/kg) followed by polish filtration and subsequent washing with ethyl alcohol (SDA 3A 200 proof, 200 mL, 5 L/kg) to give clear yellow solution. Water (deionized, 2000 mL, 20 L/kg; 1950 mL - 2100 mL) was added to the reaction solution, while the internal temperature of the reaction solution went up to 37 0C. The reaction solution was slowly cooled down to room temperature (17 - 25 0C) and stirred at ambient temperature (17 ~ 25 0C) overnight to complete crystallization. The following day (18h ~ 24h), the solids were isolated by filtration and rinsed on the filter with 30% aq. acetone (3 x 800 mL, 3 x 8 L/kg) followed by heptane (1 x 500 mL, 1 x 5 L/kg).
References:
SYNTA PHARMACEUTICALS CORP. WO2009/73147, 2009, A2 Location in patent:Page/Page column 36-37

98-88-4
575 suppliers
$10.00/5g

488832-69-5
119 suppliers
inquiry
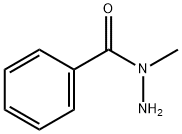
1483-24-5
34 suppliers
inquiry

488832-69-5
119 suppliers
inquiry
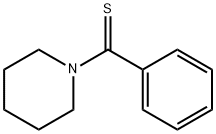
15563-40-3
5 suppliers
inquiry

488832-69-5
119 suppliers
inquiry