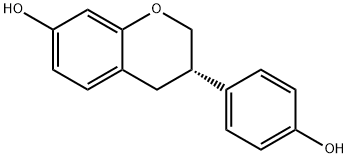
(S)-Equol synthesis
- Product Name:(S)-Equol
- CAS Number:531-95-3
- Molecular formula:C15H14O3
- Molecular Weight:242.27
![2H-1-Benzopyran, 3,4-dihydro-7-(methoxymethoxy)-3-[4-(methoxymethoxy)phenyl]-, (3S)-](/CAS/20210305/GIF/922179-56-4.gif)
922179-56-4
0 suppliers
inquiry

531-95-3
243 suppliers
$5.00/5mg
Yield:531-95-3 93%
Reaction Conditions:
with hydrogenchloride;methanol in dichloromethane at 4.8 - 20; for 6 h;
Steps:
6
Example 6; Deprotecting the bis-MOM-S-equol to S-Equol ((S)-3-(4-hydroxyphenyl)-chroman-7-ol); A solution of 42.17 g (0.128 mol) of (S)-bis-MOM-equol in 200 mL of 1:1 mixture of CH2Cl2/MeOH was placed in a 1 L 3-neck round bottom flask equipped with a magnetic stirrer, thermocouple, cooling ice bath and nitrogen line. A total of 200 mL of 10 wt % solution of HCl in MeOH (0.438 mol, 3.4 eq.) was slowly added to pre-chilled (4.8° C.) solution of bis-MOM-equol. The reaction mixture was allowed to warm up to room temperature and monitored by TLC until all starting material is converted to S-equol (Rf=0.58 for bis-MOM-equol, 0.28 for mono-MOM-equol, and 0.10 for S-equol in ethyl acetate/hexane=2:8). After 6 hours at room temperature a complete deprotection was observed. Solvent was removed under reduced pressure and precipitated solid was treated with a 500 mL of ice-cold water, extracted with ethyl acetate (2×400 mL) and combined organic phases were washed with diluted sodium bicarbonate (400 mL). Organic layer was dried over sodium sulfate and solvent volume was reduced to about 100 mL. The obtained yellowish solution was carefully diluted with 400 mL of hexane and resulted clear solution was chilled on an ice bath while stirring. Precipitated white solid was filtered off, washed with hexane (3×200 mL) and dried in a vacuum oven overnight to yield 25.24 g of s-equol as a white solid. An additional 3.54 g of the product was obtained from mother liquor. A total 28.78 g (93% isolated yield) of S-equol was obtained as a white solid with mp 162 C. Chemical HPLC and optical purity for synthesized S-equol were found to be 96.69% and 100%ee correspondingly. Reversed Phase HPLC used to determine chemical purity: Column: Waters Symmetry C18, 3.5 micron particles, 4.6×75 mm Mobile phase A: 0.1% TFA in water Mobile phase B: 0.1% TFA in acetonitrile Gradient: 5% B to 100% B in 16 minutes, return to initial conditions at 16 minutes. Detector wavelength=280 nm Injection volume=5 microliters Retention time: 7.87 min HPLC purity: 96.69% Optical purity was determine by chiral HPLC: Column: Chiracel OJ, 4.6×250 mm Isocratic, 75% (0.2% phosphoric acid in water), 25% acetonitrile Flow: 0.75 mL/min Detector wavelength: 215 nm Retention time: 54.28 min Chiral purity: 100% ee Optical rotation: [α]=-19.1° C. A reported optical rotation for S-equol crystallized from aqueous ethanol is [α]=-21.5° C. (The Merck Index, 1996, 12th edition, p 618). LC-MS traces and spectra of the S-Equol product are shown in FIGS. 6A and 6B, respectively. 1H NMR and 13C NMR data appear below. 1H NMR (300 MHz, CDCl3, δ): 9.32 (s, 1H, OH), 9.21 (s, 1H, OH), 7.09 (d, 2H, J=8.4 Hz), 6.86 (d, 1H, J=8.1 Hz), 6.72 (d, 2H, J=8.7 Hz), 6.30 (dd, 1H, J=8.4 J=2.1), 6.21 (d, 1H, J=2.4 Hz), 4.15 (ddd, 1H, CH2O, J=10.5 Hz, J=1.80 Hz), 3.88 (t, 1H, CH2O, J=10.2 Hz), 3.00 (m, 1H, CH), 2.78 (m, 2H, CH2). 13C NMR (75 MHz, CDCl3, δ): 156.515 (C7), 156.151 (C4'), 154.557 (C9), 131.711 (C10), 130.134 (C8), 128.346 (C6), 115.321 (C3'), 112.627 (C1'), 108.048 (C5), 102.547 (C2'), 70.309 (C2), 37.197 (C3), 31.324 (C4).
References:
US2007/27329,2007,A1 Location in patent:Page/Page column 10-11
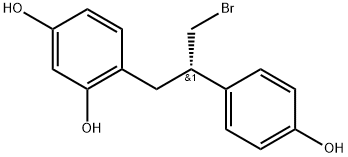
1383121-33-2
1 suppliers
inquiry

531-95-3
243 suppliers
$5.00/5mg

3722-56-3
11 suppliers
$149.90/10mg

531-95-3
243 suppliers
$5.00/5mg
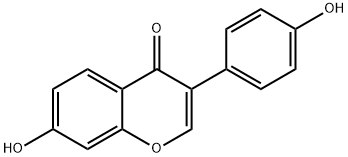
486-66-8
554 suppliers
$5.00/1g

531-95-3
243 suppliers
$5.00/5mg
![Benzene, 1-[(2S)-3-bromo-2-(4-methoxyphenyl)propyl]-2,4-dimethoxy-](/CAS/20210305/GIF/1057663-20-3.gif)
1057663-20-3
1 suppliers
inquiry

531-95-3
243 suppliers
$5.00/5mg