
erythro-L-Phenylserine synthesis
- Product Name:erythro-L-Phenylserine
- CAS Number:32946-42-2
- Molecular formula:C9H11NO3
- Molecular Weight:181.19
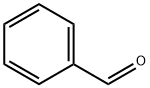
100-52-7
942 suppliers
$21.30/2g
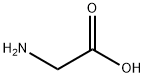
56-40-6
1212 suppliers
$5.00/25g

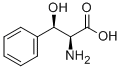
32946-42-2
10 suppliers
inquiry
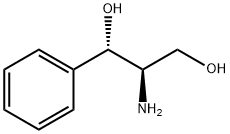
6254-48-4
52 suppliers
$125.00/250mg
Yield:> 99 % ee , > 99 % ee
Reaction Conditions:
with pyridoxal 5'-phosphate;L-allo-threonine aldolase from Thermotoga maritima in aq. phosphate buffer;dimethyl sulfoxide at 70; pH=8; for 0.333333 h;Catalytic behavior;Green chemistry;Enzymatic reaction;Overall yield = 40 %; Optical yield = 20 %de; stereoselective reaction;Aldol Condensation;Time;Reagent/catalyst;Temperature;
Steps:
Batch reactions:
Batch reactions were carried out in 20 mL test tubes at 70 °C,while stirring. The test tube was charged with 750 mg glycine(1 M), 106 mg benzaldehyde (0.1 M), 100 μL 5 mM PLP solution,2 mL DMSO, 0.9 mL (2.7 mg) TA solution (activity =0.407 U/mL) and 7 mL of a 50 mM phosphate buffer solution.The samples were collected at three different reaction times (20, 40 and 60 min). In each case 1 mL of sample was collected and the reaction was terminated by adding a 30% trichloroaceticacid solution. Then, all samples were extracted with 2 mL ofinternal standard solution (1,3-dimethoxybenzene in ethyl acetate). The enantiomeric excess (ee) and the diasteriomericexcess (de) of phenylserine were determined by HPLC analysisof the aqueous phase, while conversion of benzaldehyde wasdetermined from analysis of the organic layer using gas chromatography. Since no byproducts could be detected, the degree of conversion reflected the yield of phenylserine formation.
References:
Tibhe, Jagdish D.;Fu, Hui;Noel, Timothy;Wang, Qi;Meuldijk, Jan;Hessel, Volker [Beilstein Journal of Organic Chemistry,2013,vol. 9,p. 2168 - 2179]
![Carbamic acid, [(1S,2S)-2-hydroxy-1-(4-methyl-2,6,7-trioxabicyclo[2.2.2]oct-1-yl)-2-phenylethyl]-, 9H-fluoren-9-ylmethyl ester (9CI)](/CAS/20210111/GIF/148150-74-7.gif)
148150-74-7
0 suppliers
inquiry

32946-42-2
10 suppliers
inquiry
119364-52-2
2 suppliers
inquiry

32946-42-2
10 suppliers
inquiry
46506-67-6
0 suppliers
inquiry

32946-42-2
10 suppliers
inquiry

79898-17-2
7 suppliers
$195.00/10mg

32946-42-2
10 suppliers
inquiry

6254-48-4
52 suppliers
$125.00/250mg