Ethanol, 2-(2,2-diethoxyethoxy)- synthesis
- Product Name:Ethanol, 2-(2,2-diethoxyethoxy)-
- CAS Number:62005-55-4
- Molecular formula:C8H18O4
- Molecular Weight:178.23
Yield:62005-55-4 27%
Reaction Conditions:
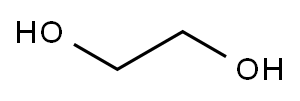
Stage #1: ethylene glycolwith potassium hydroxide at 0 - 50;
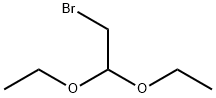
Stage #2: Bromoacetaldehyde diethyl acetal at 110; for 20 h;
Steps:
1 Step 1: Preparation of 2-(2,2-diethoxyethoxy)ethan-l-ol
A three neck flask having a content volume of 100 mL equipped with a thermometer, a stirring device and a reflux condenser was charged with ethylene glycol (9.45 g, 152.23 mmol, 8.5 mL, 2 eq). The flask was cooled on an ice bath. Potassium hydroxide (6.41 g, 114.17 mmol, 1.5 eq) was added between 0-50 °C slowly. Then 2-bromo- 1,1 -diethoxy-ethane (15 g, 76.12 mmol, 11.45 mL, 1 eq) was added and the reaction mixture was heated to 110 °C for 20 hours. The reaction mixture was cooled to 20 °C then water (30 mL) was added. The pH of the mixture was adjusted to 8 with the addition of 1 N hydrochloric acid. Then the mixture was extracted with ethyl acetate (50 mL x 5). The organic layer was dried over sodium sulfate and then concentrated under vacuum to get the residue. The residue was purified by silica gel column chromatography (5-80% ethyl acetate in petroleum ether) to get 2-(2,2-diethoxyethoxy)ethanol (3.71 g, 20.82 mmol, 27% yield) as a light yellow oil. ^-NMR (400MHz, CDC1 ,) d 4.65 (t, J = 5.2 Hz, 1H), 3.78 - 3.68 (m, 4H), 3.67 - 3.62 (m, 2H), 3.62 - 3.54 (m, 4H), 2.43 (br s, 1H), 1.24 (t, 7 = 7.1 Hz, 6H).
References:
WO2019/195609,2019,A2 Location in patent:Paragraph 001454-001455