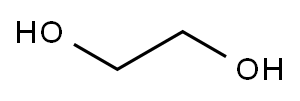
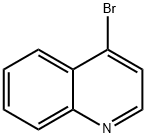
Ethanol, 2-(4-quinolinyloxy)- synthesis
- Product Name:Ethanol, 2-(4-quinolinyloxy)-
- CAS Number:24220-96-0
- Molecular formula:C11H11NO2
- Molecular Weight:189.21
Yield:24220-96-0 65%
Reaction Conditions:
with potassium carbonate;copper dichloride at 20 - 130; for 16 h;Inert atmosphere;Sealed tube;
Steps:
GeneralExperimental Procedure for the Preparation ofcompounds (6a,c-k)
General procedure: In a sealed tube, N-heteroaryl bromide 5a,c-k (10 mmol), CuCl2 (0.5 mmol) and K2CO3 (30 mmol) were charged and suspended in ethylene glycol (5 mL) and stirred at room temperature for 10 min. The blue colored suspension was refluxed at 130 °C for 16 h. The mixture was cooled to room temperature and diluted with H2O (10 mL) and extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with Brine (10 mL), dried over MgSO4, filtered and concentrated in vacuo. Purification of the crude residue was performed by column chromatography on silica gel (except for compound 6g, which was crystallized using dioxane/cyclohexane).
References:
Hamdi, Abdelrahman;Mostafa, Amany S.;Watat, Cedric Nana;Laurent, Mathieu Y.;Ben Ayed, Kawther;Selim, Khalid B.;Dujardin, Gilles [Tetrahedron Letters,2016,vol. 57,# 51,p. 5825 - 5829] Location in patent:supporting information