
Ethanone, 1-(2,3-dihydro-6-benzofuranyl)- (9CI) synthesis
- Product Name:Ethanone, 1-(2,3-dihydro-6-benzofuranyl)- (9CI)
- CAS Number:374706-07-7
- Molecular formula:C10H10O2
- Molecular Weight:162.19
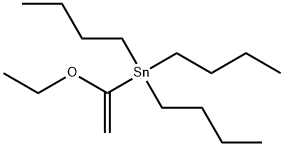
97674-02-7
218 suppliers
$15.00/1g
189035-22-1
77 suppliers
$65.00/50mg

374706-07-7
33 suppliers
$238.00/100mg
Yield:374706-07-7 73.7%
Reaction Conditions:
with bis-triphenylphosphine-palladium(II) chloride in toluene at 90; for 16 h;
Steps:
7.1 1-(2,3-dihydrobenzofuran-6-yl)ethan-1-one
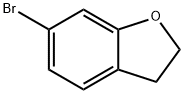
The title compound was prepared according to procedures reported in the literature and known by persons skill in the art, using 6-bromo-2,3-dihydro-1-benzofuran (1 g, 5.03 mmol) as starting material. In a preferred method, 6-bromo-2,3-dihydro-1-benzofuran (1 g, 5.03 mmol) in toluene (10 mL) was degassed for 30 min. To this solution, 1-ethoxyvinyl tributyltin (2.012 g, 5.53 mmol) and bis(triphenylphosphine)palladium dichloride (0.35 g, 0.50 mmol) were added at rt and stirred for 16 hours at 90 °C. The reaction mixture was cooled to rt and filtered through celite. After evaporation of the solvent, 6 N HCl solution in water (10 mL) was added and the mixture was stirred for 1 hour at rt. It was concentrated and neutralized with sat. NaHCO3. The desired product was extracted with DCM (50 mL), dried over Na2SO4 and concentrated. The crude product was purified by flash chromatography to give the title compound. Yield: 73.7% (0.6 g, pale yellow solid). 1H NMR (400 MHz, DMSO-d6): δ 7.48 (d, J = 7.64 Hz, 1H), 7.37-7.35 (d, J = 7.68 Hz, 1H), 7.26 (s, 1H), 4.58 (t, J = 8.76 Hz, 2H), 3.24 (t, J = 8.76 Hz, 2H), 2.53 (s, 3H). LCMS: (Method A) 163.2 (M+H), Rt. 3.01 min, 97.60% (Max).
References:
WO2017/144635,2017,A1 Location in patent:Page/Page column 32; 33

189035-22-1
77 suppliers
$65.00/50mg

374706-07-7
33 suppliers
$238.00/100mg