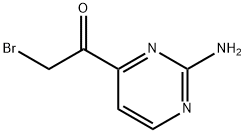
Ethanone, 1-(2-amino-4-pyrimidinyl)-2-bromo- (9CI) synthesis
- Product Name:Ethanone, 1-(2-amino-4-pyrimidinyl)-2-bromo- (9CI)
- CAS Number:106157-91-9
- Molecular formula:C6H6BrN3O
- Molecular Weight:216.04
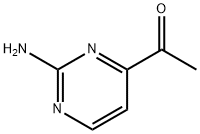
106157-82-8
70 suppliers
$80.00/100mg

106157-91-9
25 suppliers
$134.31/100mgs:
Yield:106157-91-9 65%
Reaction Conditions:
with water;hydrogen bromide;bromine in water at 20; for 15 h;
Steps:
3
To a solution of 1-(2-aminopyrimidin-4-yl)ethanone (412 mg, 3 mmol) in glacial acetic acid (1 mL) and 48% aq. HBr (0.3 mL), bromine (0.153 mL) in acetic acid (0.4 mL) was added and the resulting orange solution was stirred at room temperature for 15 hours. After diluting with ethyl acetate (15 mL), the precipitate was filtered and washed with ethyl acetate thus affording the title compound as a whitish solid (580 mg, 65%). 1H NMR (DMSO-d6/400 MHz) δ ppm: 4.9 (s, 2 H), 7.0 (d, 2 H), 8.5 (d, 2 H).
References:
US2007/142415,2007,A1 Location in patent:Page/Page column 17