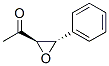
Ethanone, 1-[(2R,3S)-3-phenyloxiranyl]- (9CI) synthesis
- Product Name:Ethanone, 1-[(2R,3S)-3-phenyloxiranyl]- (9CI)
- CAS Number:190961-74-1
- Molecular formula:C10H10O2
- Molecular Weight:162.1852
Yield:50 % ee
Reaction Conditions:
with tert.-butylhydroperoxide;lanthanum(III) isopropoxide;(R)-1,1'-Bi-2-naphthol;Triphenylphosphine oxide in tetrahydrofuran;toluene at 30; for 96 h;Molecular sieve;Inert atmosphere;Schlenk technique;Overall yield = 29 %; enantioselective reaction;Reagent/catalyst;
Steps:
General procedure for the catalytic asymmetric homogeneous epoxidations of enones
General procedure: Under an argon atmosphere, a Schlenk flask was charged with pre-dried 4? molecular sieves (400.0 mg), the ligand (0.05 mmol), Ln(OiPr)3 (0.05 mmol), Ph3P(O) (84.0 mg, 0.30 mmol) and THF (0.75 mL) freshly distilled on Na/benzophenone. The solution was stirred for 1 hour at 30°C. Then, a solution of anhydrous TBHP (215 μL, 1.00 mmol, 4.7 M in toluene) was added. After stirring at 30°C for 1 hour, enone (0.50 mmol) was added. Stirring was maintained at 30°C for 2 to 7 days. Then, the reaction mixture was hydrolyzed with a saturated NH4Cl solution, the organic phase was extracted with ethyl acetate, dried over Na2SO4 and concentrated. The conversion and the enantiomeric excesses were determined by HPLC. HPLC analysis of trans-epoxy-1,3-diphenylpropan-1-one, tR = 87.5 min (2S,3R)-isomer (major) and 91.0 min (2R,3S)-isomer (minor). HPLC analysis of trans-epoxy-4-phenylbutan-2-one, tR = 42.0 min (3R,4S)-isomer (minor) and 50.0 min (3S,4R)-isomer (major).
References:
El Kadiri, Moulay Youness;Framery, Eric;Andrioletti, Bruno [Tetrahedron Letters,2012,vol. 53,# 47,p. 6335 - 6338] Location in patent:supporting information
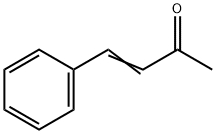
122-57-6
407 suppliers
$5.00/25g
![Ethanone, 1-[(2R,3S)-3-phenyloxiranyl]- (9CI)](/CAS/GIF/190961-74-1.gif)
190961-74-1
2 suppliers
inquiry
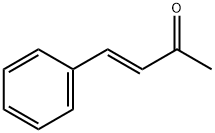
1896-62-4
221 suppliers
$11.00/100g
![Ethanone, 1-[(2R,3S)-3-phenyloxiranyl]- (9CI)](/CAS/GIF/190961-74-1.gif)
190961-74-1
2 suppliers
inquiry

100-52-7
941 suppliers
$20.37/250g
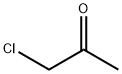
78-95-5
4 suppliers
$21.50/5G
![Ethanone, 1-[(2S,3R)-3-phenyloxiranyl]- (9CI)](/CAS/GIF/146388-50-3.gif)
146388-50-3
2 suppliers
inquiry
![Ethanone, 1-[(2R,3S)-3-phenyloxiranyl]- (9CI)](/CAS/GIF/190961-74-1.gif)
190961-74-1
2 suppliers
inquiry