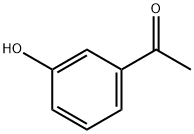
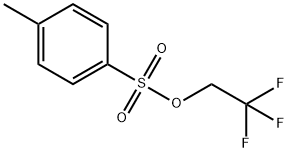
Ethanone, 1-[3-(2,2,2-trifluoroethoxy)phenyl]- synthesis
- Product Name:Ethanone, 1-[3-(2,2,2-trifluoroethoxy)phenyl]-
- CAS Number:1017025-91-0
- Molecular formula:C10H9F3O2
- Molecular Weight:218.17
Yield:1017025-91-0 71%
Reaction Conditions:
Stage #1: 3-Hydroxyacetophenonewith sodium hydride in N,N-dimethyl-formamide at 20; for 0.166667 h;
Stage #2: 2,2,2-Trifluoroethyl p-toluenesulfonate in N,N-dimethyl-formamide at 130; for 15 h;
Steps:
3.4. General Procedure for Fluoroethoxylation of Hydroxyacetophenones and benzaldehydes
General procedure: A round bottom flask was charged with sodium hydride (1.2 eq.). The appropriate hydroxyacetophenone or hydroxybenzaldehyde (1 eq.) in DMF (100 mL) was added dropwise over 10 min at rt. Then 2,2,2-trifluoroethyl-4-methylbenzenesulphonate (1.2 eq.) in DMF (50 mL) was added dropwise. The reaction mixture was then allowed to reflux at 130°C for 15 h. After completion of reaction, monitored by TLC, the reaction mixture was poured into water and extracted with ethyl acetate. The extract was washed with water, brine solution and finally dried over sodium sulfate and evaporated to dryness. The crude reaction mixture was purified by column chromatography (silica gel 60-200 mesh) to afford desired compounds.
References:
Devi, Kavita;Rajendran, Vinoth;Ayushee;Rangarajan;Singh, Rishi Pal;Ghosh, Prahlad C.;Singh, Manjula [Molecules,2018,vol. 23,# 5,art. no. 1174]