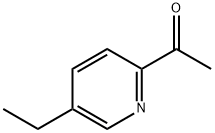
Ethanone,1-(5-ethyl-2-pyridinyl)- synthesis
- Product Name:Ethanone,1-(5-ethyl-2-pyridinyl)-
- CAS Number:286411-85-6
- Molecular formula:C9H11NO
- Molecular Weight:149.19
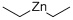
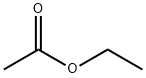
557-20-0
228 suppliers
$14.00/1g
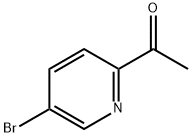
214701-49-2
190 suppliers
$24.00/1g

286411-85-6
7 suppliers
inquiry
Yield:286411-85-6 88%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in 1,4-dioxane at 50; for 1 h;Inert atmosphere;
Steps:
1-(5-Ethyl-2-pyridinyl)ethanone 18.
A mixture of 17 (12.6 g, 63 mmol) and [1,1’-bis(diphenylphosphino)ferrocene]dichloropalladium(II) (1.20 g, 1.52 mmol) in dioxane (240mL) was degassed by sparging with nitrogen for 20 minutes. To this mixture was added a solution of diethyl zinc (50mL, 15% w/w) over 30 minutes. The mixture was then warmed to 50 °C and the orange mixture gave way to a dark orange solution and yellow solids as it stirred at 50°C for 60 minutes. The mixture was cooled to room temperature and was cast into EtOAc (400mL) and water (400mL). The organic phase was separated, the aqueous phase was extracted with EtOAc (2x250mL). The combined organic phases were washed with brine (1000mL), dried (Na2SO4), filtered and concentrated in vacuo to furnish crude 18 as a brown oil. Crude 18 was purified by distillation (BP = 54-55 °C, 0.32mm Hg) to give 18 (8.27g, 88%) as a very pale yellow oil.1H NMR (400 MHz, CDCl3): δ = 8.50 (d, J = 1.9 Hz, 1), 7.96 (d, J = 7.9 Hz, 1), 7.63 (dd, J = 7.9, 1.9 Hz, 1), 2.71 (q, J = 7.6 Hz, 2), 2.69 (s, 3), 1.27 (t, J = 7.6 Hz, 3); MS (ESI+) m/z 150.1 (M + H).
References:
Tanis, Steven P.;Colca, Jerry R.;Parker, Timothy T.;Artman, Gerald D.;Larsen, Scott D.;Gadwood, Robert C.;Zeller, James R. [Tetrahedron Letters,2019,vol. 60,# 33,art. no. 150931] Location in patent:supporting information
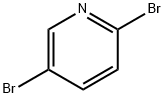
624-28-2
714 suppliers
$5.00/5g

286411-85-6
7 suppliers
inquiry
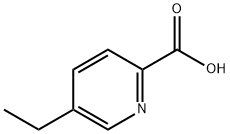
770-08-1
90 suppliers
$140.00/1g

286411-85-6
7 suppliers
inquiry