Ethenamine, N,N-dimethyl-2-(3-nitro-2-pyridinyl)- synthesis
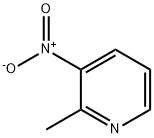
- Product Name:Ethenamine, N,N-dimethyl-2-(3-nitro-2-pyridinyl)-
- CAS Number:65156-92-5
- Molecular formula:C9H11N3O2
- Molecular Weight:193.2
Yield:65156-92-5 73%
Reaction Conditions:
in N,N-dimethyl-formamide at 90; for 4 h;Inert atmosphere;
Steps:
E.c (c)
(c)
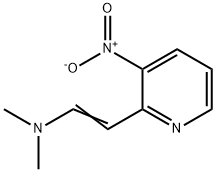
(E)-N,N-Dimethyl-2-(3-nitropyridin-2-yl)ethenamine
A modified procedure of Cash et al. (Org. Biomol. Chem. 2005, 3. 3701-3706) was used. 2-Methyl-3-nitropyridine (0.7 g, 5.1 mmol) was dissolved in 15 ml dry DMF and stirred under nitrogen.
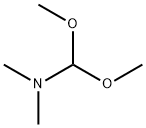
Dimethylformamiddimethylacetal (DMF-DMA) (1.35ml, 1.22g, 10.2 mmol) was added dropwise.
The reaction was heated to 90°C for 4 h.
After approximately 15 min a deep redish color appeared.
After evaporating the solvent (E)-N,N-Dimethyl-2-(3-nitropyridin-2-yl)ethenamine is obtained as a red oil (0.16 g, 0.8 mmol, 73%) which can be used without further purification. 1H NMR (300 MHz, CDCl3) 8.40 (pdd; 3J = 1.7 Hz; 3J = 4.4 Hz; 1H; H-6); 8.16 (pdd; 3J = 1.7 Hz; 3J = 8.3 Hz; 1H; H-4) ; 8.04 (d; 3JAX = 12.5 Hz; 1H; H-8) ; 6.76 (pdd; 3J = 4.4 Hz; 3J = 8.3 Hz; 1H; H-5); 6.15 (d; 3JAX = 12.5 Hz; 1H; H-7); 3.01 (s; 6H; CH3).
References:
EP2512469,2016,B1 Location in patent:Paragraph 0075; 0076
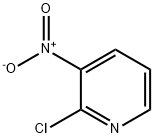
5470-18-8
447 suppliers
$6.00/5g

65156-92-5
5 suppliers
inquiry
![ALPHA-[2-(3'-NITROPYRIDINYL)] DIETHYL MALONATE](/CAS/GIF/64362-41-0.gif)
64362-41-0
35 suppliers
$306.89/250mg

65156-92-5
5 suppliers
inquiry