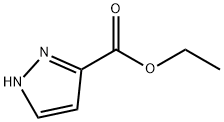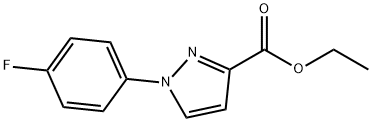
ethyl 1-(4-fluorophenyl)-1H-pyrazole-3-carboxylate synthesis
- Product Name:ethyl 1-(4-fluorophenyl)-1H-pyrazole-3-carboxylate
- CAS Number:115342-25-1
- Molecular formula:C12H11FN2O2
- Molecular Weight:234.23
Yield:115342-25-1 77%
Reaction Conditions:
with copper(l) iodide;potassium carbonate;(1S,2S)-N,N'-dimethyl-1,2-diaminocyclohexane in toluene at 110; for 2 h;
Steps:
97 Synthesis of ethyl 1-(4-fluorophenyl)pyrazole-3-carboxylate
To a solution of 1-fluoro-4-iodo-benzene (1.47 g, 6.6 mmol, 1.3 eq) and ethyl 1H-pyrazole-3-carboxylate (0.71 g, 5.1 mmol, 1 eq) in 15 mL of toluene was added CuI (0.19 g, 1.0 mmol, 0.2 eq), trans-N,-N-dimethylcyclohexane 1,2-diamine (0.4 mL, 2.5 mmol, 0.2 eq), and potassium carbonate (1.4 g, 10 mmol, 2 eq).
The reaction mixture was heated at 110° C. for 2 d then filtered and concentrated.
The residue was purified by silica gel column chromatography (hex:EtoAc 4:1) to afford ethyl 1-(4-fluorophenyl)pyrazole-3-carboxylate (0.92 g, 3.9 mmol, 77%).
References:
US2014/154179,2014,A1 Location in patent:Paragraph 0388; 0389
121-46-0
152 suppliers
$30.00/10g
![Ethylchloro[(4-fluorophenyl)hydrazono]acetate](/CAS/20200401/GIF/37522-19-3.gif)
37522-19-3
18 suppliers
$192.00/1g

115342-25-1
13 suppliers
inquiry