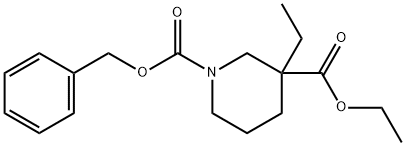
Ethyl 1-Cbz-3-ethylpiperidine-3-carboxylate synthesis
- Product Name:Ethyl 1-Cbz-3-ethylpiperidine-3-carboxylate
- CAS Number:1338930-81-6
- Molecular formula:C18H25NO4
- Molecular Weight:319.4
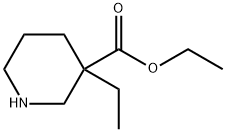
170843-44-4
1 suppliers
inquiry
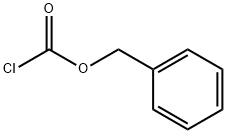
501-53-1
412 suppliers
$10.00/1g

1338930-81-6
14 suppliers
$438.00/10g
Yield:1338930-81-6 7.28 g
Reaction Conditions:
with triethylamine in tetrahydrofuran at 0 - 20;Inert atmosphere;
Steps:
3.1. 1-Benzyl 3-ethyl 3-ethylpiperidine-1,3-dicarboxylate (10b)
The 3-ethyl 3-ethylpiperidin carboxylate 9b was synthesized as reported in the literature.19 To a solution of ethyl nipecotate 8 (7.00 g; 44.6 mmol) in THF (10 mL), was added dropwise sodium hexamethyl disilazane (NaHMDS) in THF (1.05 M, 50 ml) at -20 °C to -10 °C, and the resulting solution was stirred for 1 h. EtI (6.95 g; 44.6 mmol) was then added to the solution while keeping the temperature at -10 °C to -20 °C, and the resulting mixture was allowed to warm to room temperature without the cold bath. The reaction mixture was next quenched by H2O and separated, and the organic layer was dried over Na2SO4 and concentrated to afford the intermediate 9b (8.20 g), which was used in the next reaction without purification. To a solution of 9b and triethylamine (TEA) (6.75 g) in THF was slowly added carbobenzyloxy chloride (CbzCl) (6.4 ml) at 0 °C, and the resulting mixture was allowed to warm to room temperature with the ice-bath. The reaction mixture was then filtered through Celite, washed with satd NH4Cl aq and brine, dried over Na2SO4, and the filtrate was concentrated. The residue was purified by SiO2 column chromatography (Hexane/EtOAc = 10/1 to 5/1) to give 7.28 g (51%) of 10b as a white solid. 1H NMR (400 MHz, CDCl3) δ: 0.82 (3H, bs), 1.18 (3H, br s), 1.40-1.70 (5H, m), 2.04-2.10 (1H, m), 3.10-3.25 (2H, m), 3.60-3.65 (1H, m), 4.00-4.18 (3H, m), 5.12 (2H, s), 7.27-7.40 (5H, m). LRMS (ESI+): m/z 414.2.
References:
Nishio, Yukihiro;Kimura, Hidenori;Sawada, Naoyuki;Sugaru, Eiji;Horiguchi, Masakuni;Ono, Michiko;Furuta, Yudai;Sakai, Mutsuko;Masui, Yumi;Otani, Misato;Hashizuka, Takahiko;Honda, Yayoi;Deguchi, Jiro;Nakagawa, Tsutomu;Nakahira, Hiroyuki [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 18,p. 5490 - 5499] Location in patent:experimental part

71962-74-8
49 suppliers
inquiry

1338930-81-6
14 suppliers
$438.00/10g