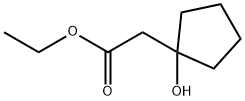
Ethyl (1-hydroxycyclopentyl)acetate synthesis
- Product Name:Ethyl (1-hydroxycyclopentyl)acetate
- CAS Number:3197-76-0
- Molecular formula:C9H16O3
- Molecular Weight:172.22
Yield:3197-76-0 99%
Reaction Conditions:
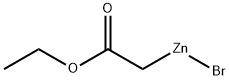
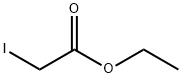
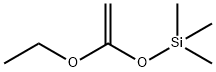
Stage #1: ethyl bromoacetatewith chloro-trimethyl-silane;zinc in diethyl ether at 20; for 2 h;Reflux;Reformatsky reaction;
Stage #2: cyclopentanone in diethyl ether at 19 - 21; for 1 h;Reformatsky reaction;
Stage #3: with ammonia in diethyl ether;water;Cooling with ice;
Steps:
5.1.39. Ethyl 1-hydroxycyclopentylacetate (51)
Trimethylchlorosilane (3.60 mL, 28.2 mmol) was added from a syringe to a suspension of zinc powder (25.2 g, 0.385 mol) in Et2O (500 mL). The mixture was stirred for 15 min at room temperature and then heated to reflux. The heating was stopped, and ethyl bromoacetate (32.4 mL, 0.292 mol) was added at such a rate that the ether boiled gently. After being heated to reflux for 1 h, the mixture was stirred for 1 h at room temperature. A solution of 49 (20.2 g, 0.240 mol) in Et2O (30 mL) was added while the temperature of the mixture was maintained at 19-21 °C by intermittent cooling. After being stirred for 1 h at room temperature, the mixture was poured into iced 25% ammonia (500 mL). The aqueous phase was extracted with ether, the combined phases were dried on K2CO3 and evaporated, and 51 was obtained as a colorless oil (41.0 g, 99%). 1H NMR (400 MHz, CDCl3) δ 1.13 (3H, t, J = 7.3 Hz), 1.34-1.52 (4H, m), 1.61-1.75 (4H, m), 2.45 (2H, s), 3.22 (1H, s), 4.03 (2H, q, J = 7.3 Hz). HRMS (CI+) for C9H17O3 [M+H]+: calcd, 173.1178; found, 173.1181.
References:
Seto, Shigeki;Yumoto, Kazuhiko;Okada, Kyoko;Asahina, Yoshikazu;Iwane, Aya;Iwago, Maki;Terasawa, Reiko;Shreder, Kevin R.;Murakami, Koji;Kohno, Yasushi [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 3,p. 1188 - 1200] Location in patent:experimental part
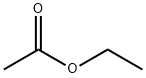
141-78-6
606 suppliers
$22.37/250ml

120-92-3
400 suppliers
$11.00/25g

3197-76-0
12 suppliers
inquiry