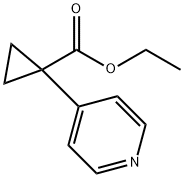
Ethyl 1-(pyridin-4-yl)cyclopropanecarboxylate synthesis
- Product Name:Ethyl 1-(pyridin-4-yl)cyclopropanecarboxylate
- CAS Number:858035-95-7
- Molecular formula:C11H13NO2
- Molecular Weight:191.23
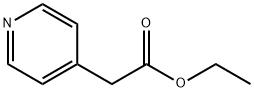
54401-85-3
110 suppliers
$17.67/1gm:

106-93-4
377 suppliers
$15.00/5g

858035-95-7
16 suppliers
inquiry
Yield:858035-95-7 82%
Reaction Conditions:
Stage #1: pyridin-4-yl-acetic acid ethyl esterwith lithium hexamethyldisilazane in tetrahydrofuran;N,N-dimethyl-formamide at 25; for 0.5 h;
Stage #2: ethylene dibromide in tetrahydrofuran;N,N-dimethyl-formamide at 25; for 2 h;
Steps:
1.1 1.1 1 -Pyridin-4-yl-cyclopropanecarboxylic acid ethyl ester
Pyridin-4-yl-acetic acid ethyl ester (2.00 g; 12.107 mmol) was dissolved in DMF (30.0 mL), lithium bis(trimethylsilyl)amide (1 M in THF; 18.16 mL; 18.161 mmol) was added and the mixture was stirred at 25 °C for 30 min. 1,2- Dibromoethane (1.25 mL; 14.529 mmol) was added and the mixture was stirred at 25 °C for 1 h. Lithium bis(trimethylsilyl)amide (1 M in THF; 18.16 mL; 18.161 mmol was added and the mixture stirred for another 1 h. The mixture was quenched under ice cooling with acetic acid (4 mL) and evaporated. The residue was partitioned between NH4CI solution and dichloromethane. The organic phase was separated and the water layer extracted twice with dichloromethane. The combined organic phases were dried over MgSO4 and evaporated under reduced pressure. The residue was purified by RP-flash chromatography (Isco Companion). Yield: 1.90 g (82%) brown solid; HPLC/MS, Rt: 0.94 min; (M+H) 192.1 ; 1H NMR (400 MHz, DMSO-d6/TFA) δ 8.96-8.84 (m, 2H), 8.17-8.08 (m, 2H), 4.14 (q, J = 7.1Hz, 1H), 1.81 -1.77 (m, 1H), 1.56-1.48 (m, 1H), 1.17 (t, J = 7.1Hz, 3H).
References:
WO2017/167676,2017,A1 Location in patent:Page/Page column 40
1069115-10-1
12 suppliers
inquiry

858035-95-7
16 suppliers
inquiry