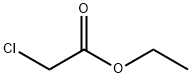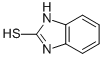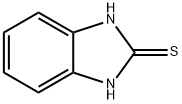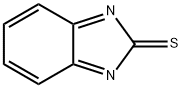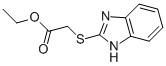
Ethyl=(1H-benzimidazol-2-ylthio)acetate synthesis
- Product Name:Ethyl=(1H-benzimidazol-2-ylthio)acetate
- CAS Number:5429-62-9
- Molecular formula:C11H12N2O2S
- Molecular Weight:236.294
Yield:5429-62-9 87%
Reaction Conditions:
with potassium carbonate in acetone; for 6 h;Reflux;
Steps:
Ethoxycarbonylmethyl-2-mercaptobenzimidazole(3)22.
A mixture of 2-mercaptobenzimidazole 2(15g, 0.1 mole), dry acetone (300 ml), anhydrous K2CO3 (13.8 g, 0.1 mole) and ethylchloroacetate (15.92 g, 0.13 mole) was heated under reflux for 6 h(TLC). The reaction mixture was filtered of and the filtrate was evaporated under reduces pressure. The residue was recrystallized from ethanol to yield brown oil in 87% yield. Rf = 0.48 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): d = 1.26 (t, 3H, J = 8.1Hz, CH3CH2), 4.15 (q, 2H, J = 8.1 Hz, CH3CH2),4.55 (s, 2H, CH2), 5.25 (brs, 1H, NH), 7.32 (d, 2H, J= 5.5Hz, H-2), 7.57 (d, 2H, J = 5.5 Hz, H-3).
References:
Amer, Hamada H.;Ali, Omar M.;El-Kafaween, Ibrahim Kh. [Oriental Journal of Chemistry,2017,vol. 33,# 5,p. 2303 - 2310]