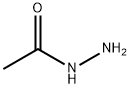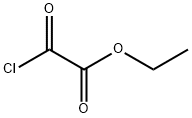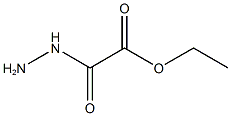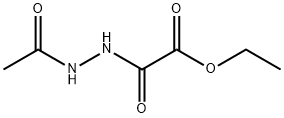
Ethyl 2-(2-acetylhydrazinyl)-2-oxoacetate synthesis
- Product Name:Ethyl 2-(2-acetylhydrazinyl)-2-oxoacetate
- CAS Number:42042-84-2
- Molecular formula:C6H10N2O4
- Molecular Weight:174.15
Yield:42042-84-2 94%
Reaction Conditions:
with triethylamine in dichloromethane at 20;Cooling with ice;
Steps:
8 preparation of compound 8-4
To a solution of compound 8-2 (1.0 g, 13 mmol) in DCM (20 mL) was added triethylamine (2 mL, 14.3 mmol) in an ice bath, and then compound 8-3 (1.8 g, 13 mmol) was added dropwise slowly. After the addition, the mixture was stirred at rt overnight. After the reaction was complete, the mixture was diluted with EtOAc (100 mL) and filtered to remove salt. The filtrate was concentrated in vacuo. The residue was purified by silica gel column chromatography eluted with PE : EtOAc (V : V) = 5 : 1 to afford a white solid 8-4 (2.2 g, 13 mmol, 94%). MS (ESI, neg.ion ) m/z: 173[M-1]
References:
WO2016/127859,2016,A1 Location in patent:Paragraph 00192