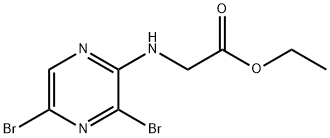
ethyl 2-(3,5-dibromopyrazin-2-ylamino)acetate synthesis
- Product Name:ethyl 2-(3,5-dibromopyrazin-2-ylamino)acetate
- CAS Number:1228013-60-2
- Molecular formula:C8H9Br2N3O2
- Molecular Weight:338.98
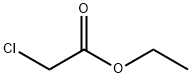
105-39-5
379 suppliers
$10.00/5g

24241-18-7
421 suppliers
$6.00/5g

1228013-60-2
10 suppliers
inquiry
Yield:1228013-60-2 57%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-formamide at 0 - 76;
Steps:
6.B
A 2000 mL 3-necked round bottomed flask was charged with 2-amino-3,5-dibromopyrazine (172 g, 680 mmol) in dimethylformamide (860 mL) and cooled to 0-5 0C. Cesium carbonate (288 g, 884 mmol) was added in one portion followed by the portion- wise addition of ethyl chloroacetate (87 mL, 816 mmol). The solution was allowed to warm to 20-25 0C then heated to 55 0C (exotherm observed, max temperature observed 76 0C). Once the internal reaction temperature subsided to 65 0C the reaction was heated at 65 0C for ~4 h. The reaction was cooled to 20-25 0C and filtered through filter paper to remove inorganic salts and the solid was washed with dimethylformamide (3 vol). The filtrate was added dropwise to 16 vol of ice-water (8 vol ice/8 vol water) and the slurry was allowed to agitate for 12 -24 h. The resulting brown solid was isolated following filtration and washed with water (10 vol) and air-dried. Crude product was dissolved in methyl t-butyl ether (3.46 L, 15 vol). Charcoal (C-906 from Ecosorb, 20 wt%, 46.1 g) was added and the mixture was heated at reflux for 1 h. After cooling to rt, the charcoal was removed over a Celite bed and the filtrate was concentrated to dryness. The crude was dissolved in ethyl acetate (576 mL, 2.5 vol) and concentrated to a thick slurry. A solution of 2 % ethyl acetate in heptane (1.15 L, 5 vol) was added and the mixture was stirred at rt for 30-60 min. The product was collected by filtration, washed with heptane (2-3 vol) and dried under high vacuum at 35-40 0C for 16 h to afford the desired compound as an off-white solid (109 g, 47 % yield). A second crop was isolated from the mother liquor as follows: the filtrate was concentrated to give a crude oil. Ethyl acetate (1 vol.) was added. The resulting solution was seeded with previously isolated product and cooled at 0-5 0C for 1 h. The resulting solid was collected by filtration and washed with cold ethyl acetate: heptane (1 :1 mixture, <1 vol). The solid was dried as described previously and combined with the first crop to provide the title compound (132 g, 57 % total yield). MS (ESI) m/z 337.8 [M-I]+, 339.8 [M+l]+, 341.8 [M+3]+.
References:
WO2010/62571,2010,A1 Location in patent:Page/Page column 96-97
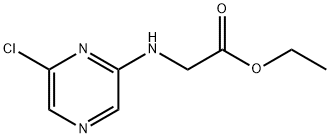
1228013-59-9
2 suppliers
inquiry

1228013-60-2
10 suppliers
inquiry
