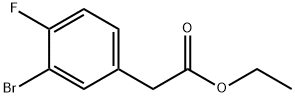
ETHYL 2-(3-BROMO-4-FLUOROPHENYL)ACETATE synthesis
- Product Name:ETHYL 2-(3-BROMO-4-FLUOROPHENYL)ACETATE
- CAS Number:1228689-75-5
- Molecular formula:C10H10BrFO2
- Molecular Weight:261.09
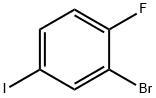
811842-30-5
133 suppliers
$6.00/1g
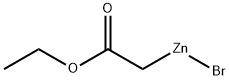
5764-82-9
23 suppliers
inquiry

1228689-75-5
8 suppliers
$394.00/1g
Yield:1228689-75-5 71%
Reaction Conditions:
with 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene;bis(dibenzylideneacetone)-palladium(0) in tetrahydrofuran at 65; for 4 h;Inert atmosphere;Sealed tube;chemoselective reaction;Reformatsky Reaction;
Steps:
4.3 General procedure for the Reformatsky-Negishi coupling employing ethyl 2-bromozincacetate (2a)
General procedure: To a 20 mL vial with a stir bar was added aryl halide 1 (2.00 mmol), Pd(dba)2 (28.8 mg, 2.5 mol %), Xantphos (28.9 mg, 2.5 mol %). The vial was sealed with a Teflon-lined cap and THF (6.0 mL) was added. The mixture was vacuumed and backfilled with nitrogen (3×). A solution of ethyl 2-bromozincacetate (2a) in THF (0.40 M, 6.0 mL, 1.2 equiv) filtered through a Target Nylon 0.45 μm filter (1.25-inch OD) was syringed in and the reaction mixture was then heated to 65 °C and monitored by HPLC. Upon reaction completion based on HPLC analysis (≥95% conversion unless the reaction was stalled), the mixture was cooled to room temperature and quenched with 1 M aq HCl (5.0 mL), followed by addition of brine (5.0 mL). The organic layer was separated and concentrated in vacuum. The residue was purified by silica gel column chromatography using gradient EtOAc in hexanes.
References:
Wong, Brian;Linghu, Xin;Crawford, James J.;Drobnick, Joy;Lee, Wendy;Zhang, Haiming [Tetrahedron,2014,vol. 70,# 7,p. 1508 - 1515]