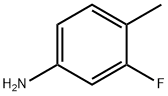
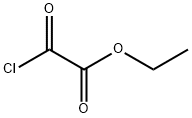
ethyl 2-(3-fluoro-4-methylanilino)-2-oxoacetate synthesis
- Product Name:ethyl 2-(3-fluoro-4-methylanilino)-2-oxoacetate
- CAS Number:69066-06-4
- Molecular formula:C11H12FNO3
- Molecular Weight:225.22
Yield:69066-06-4 94%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 0 - 20; for 6 h;
Steps:
1.2 4.1.2. Ethyl 2-((3,4-dichlorophenyl)amino)-2-oxoacetate (6b)
General procedure: To a stirred solution of 3,4-dichloroaniline 4b (1.94 g, 12.0 mmol) in THF (20.0 mL) were added ethyl chloroglyoxylate (1.11 mL, 10.0 mmol) and Et3N (15.2 mL, 11.0 mmol) at 0 °C. The mixture was stirred at room temperature for 6 h. After the precipitate was filtrated off, the filtrate solution was concentrated under reduced pressure. The residue was dissolved in EtOAc, and washed with 1.0 M HCl, saturated NaHCO3 and brine, then dried over MgSO4. Concentration under reduced pressure to provide the title compound 6b (1.58 g, 95% yield) as white powder, which was used without further purification.
References:
Narumi, Tetsuo;Arai, Hiroshi;Yoshimura, Kazuhisa;Harada, Shigeyoshi;Hirota, Yuki;Ohashi, Nami;Hashimoto, Chie;Nomura, Wataru;Matsushita, Shuzo;Tamamura, Hirokazu [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 9,p. 2518 - 2526]