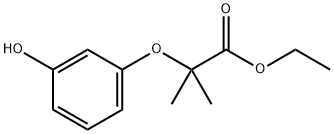
ethyl 2-(3-hydroxyphenoxy)-2-methylpropanoate synthesis
- Product Name:ethyl 2-(3-hydroxyphenoxy)-2-methylpropanoate
- CAS Number:328919-24-0
- Molecular formula:C12H16O4
- Molecular Weight:224.25
Yield:328919-24-0 72%
Reaction Conditions:
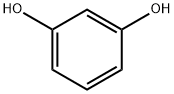
Stage #1: recorcinolwith ethanol;sodium for 1 h;Heating / reflux;
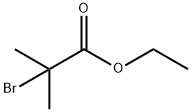
Stage #2: ethyl 2-bromoisobutyrate in ethanol; for 3 h;Heating / reflux;
Stage #3: with water;acetic acid
Steps:
10.b
b) Ethyl 2-(3-hydroxy-phenoxy)-2-methyl-propionate (10b); 15 g of resorcinol (136 mmol) are added to 120 ml of a solution of sodium (6.3 g, 274 mmol) in ethanol. The mixture is placed at reflux for 1 h then a solution of ethyl bromoisobutyrate (13.2 ml, 90 mmol) in 30 ml of ethanol is added dropwise. Heating is maintained for 3 h then the reaction medium is dry concentrated. The residue obtained is taken up in a solution of water and acetic acid then extracted with AcOEt. The organic phases are washed with water, dried on MgSO4, then dry concentrated. The residue obtained is purified by flash chromatography on silica (CH2Cl2:AcOEt 90:10). 14.4 g of oil corresponding to intermediate 10b are thus isolated (72% yield). TLC silica gel 60 F 254 Merck, CH2Cl2:AcOEt 70:30, Rf=0.66.
References:
US2008/167313,2008,A1 Location in patent:Page/Page column 15