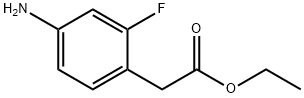
Ethyl 2-(4-aMino-2-fluorophenyl)acetate synthesis
- Product Name:Ethyl 2-(4-aMino-2-fluorophenyl)acetate
- CAS Number:73781-63-2
- Molecular formula:C10H12FNO2
- Molecular Weight:197.21
914224-31-0
44 suppliers
$87.00/100mg

64-17-5
705 suppliers
$10.00/50g

73781-63-2
34 suppliers
$50.00/100mg
Yield:73781-63-2 93%
Reaction Conditions:
Stage #1: 2-(4-amino-2-fluorophenyl)acetic acid;ethanolwith thionyl chloride at 0 - 40; for 48 h;
Stage #2: with sodium hydrogencarbonate in dichloromethane;water;Product distribution / selectivity;
Steps:
4.a
Thionyl chloride (986 mL, 8.51 mol) was added dropwise to a stirred suspension of 2-(4-amino-2-fluorophenyl)acetic acid (960 g, 5.68 mol) in ethanol (10 L) at 00C under a nitrogen atmosphere. The reaction mixture was then heated to 400C for 48 hours, with the reaction progress monitored by 1H-nmr spectroscopy. The reaction mixture was evaporated to dryness and the residue redissolved in a mixture of dichloromethane (8 L) and saturated sodium hydrogen carbonate solution (5 L). Further sodium hydrogen carbonate solution was added (~8 L) until the aqueous phase was basic. The layers were separated and the organic phase washed with saturated sodium hydrogen carbonate solution (4 L), dried over magnesium sulphate and the solvent removed under reduced pressure gave the desired product, ethyl (4-amino-2-fluorophenyl)acetate, as an orange oil (1042 g, 93% yield), that may crystallise upon standing.
References:
WO2007/88190,2007,A1 Location in patent:Page/Page column 58

369-34-6
408 suppliers
$5.00/5g

73781-63-2
34 suppliers
$50.00/100mg