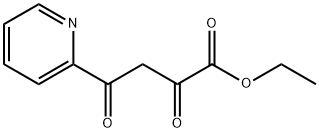
ETHYL 2,4-DIOXO-4-(2-PYRIDINYL)BUTANOATE synthesis
- Product Name:ETHYL 2,4-DIOXO-4-(2-PYRIDINYL)BUTANOATE
- CAS Number:92288-93-2
- Molecular formula:C11H11NO4
- Molecular Weight:221.21
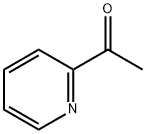
1122-62-9
546 suppliers
$7.00/10g
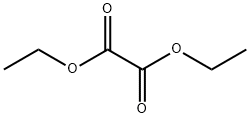
95-92-1
408 suppliers
$5.00/5G

92288-93-2
34 suppliers
$118.00/500mg
Yield:92288-93-2 41%
Reaction Conditions:
Stage #1: 2-acetylpyridinewith sodium hydride in N,N-dimethyl-formamide at 0 - 20; for 0.583333 h;
Stage #2: oxalic acid diethyl ester in N,N-dimethyl-formamide at 0 - 20; for 18.1667 h;
Stage #3: with water in diethyl ether;N,N-dimethyl-formamide;
Steps:
3
[Referential Example 3] Ethyl 4-(2-pyridyl)-2,4-dioxobutanoate; [Show Image] Under argon atmosphere, 2-acetylpyridine (1.39 ml) was added dropwise to a suspension of 60% sodium hydride (0.991 g) in N, N-dimethylformamide (30 ml), and the mixture was stirred for 5 minutes at 0°C and for another 30 minutes at room temperature. To the reaction liquid was added dropwise diethyl oxalate (3.36 ml) at 0°C, and the mixture was stirred for 10 minutes and for another 18 hours at room temperature. To the reaction liquid were added water and diethylether, and the aqueous layer was separated. The aqueous layer was neutralized with 1N aqueous hydrochloric acid (24.8 ml), and the solution was extracted with ethyl acetate. The organic layer was washed twice with water, and dried over anhydrous sodium sulfate. After filtration, the solvent was evaporated under reduced pressure, and the residue was purified by column chromatography on silica gel (methanol-chloroform) to give the title compound (1.12 g, 41%) as a solid. 1H-NMR (400MHz, CDCl3)δ: 1.40-1.43 (3H, m), 4.38-4.43 (2H, m), 7.51-7.54 (1H, m), 7.62 (1H, s), 7.89-7.93 (1H, m), 8.18 (1H, d, J = 8.0 Hz), 8.73 (1H, d, J = 4.4 Hz). MS (EI)m/z: 221 (M+).
References:
EP1698626,2006,A1 Location in patent:Page/Page column 20-21