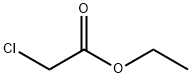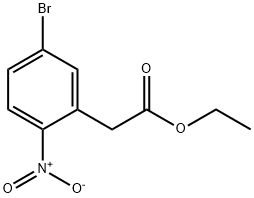
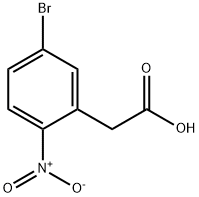
ethyl 2-(5-bromo-2-nitrophenyl)acetate synthesis
- Product Name:ethyl 2-(5-bromo-2-nitrophenyl)acetate
- CAS Number:870274-21-8
- Molecular formula:C10H10BrNO4
- Molecular Weight:288.09
124840-61-5
43 suppliers
$40.00/100mg

870274-21-8
11 suppliers
inquiry
Yield:870274-21-8 99.2%
Reaction Conditions:
with sulfuric acid in ethanol;Reflux;
Steps:
To a solutuion of 60 ml 6 N HCl and 60 ml AcOH, dissove 17.9 g 2 (50 mmol) and stir at 110 oC overnight. Romve all sovent and obtain 12.5 g 5-Bromo-2-Nitrophenylacetic acid (3), mp. 174.4-175.9 oC, yield 98%. Intermediate 3 proceed to next step without further purification. To a solution of 100 ml dehydrated EtOH and 0.5 ml conc. H2SO4, add 12.9 g 3 (50 mmol).Ethyl 2-(5-Bromo-2-Nitrophenyl)Acetate (4). Put the mixture in oil bath and reflux overnight. Cool down to rt and place at -5 oC overnight until the total precipitation occured. Filtrate white precipitate and obtain 12.5 Ethyl 2-(5-Bromo-2-Nitrophenyl)Acetate (4), yield 99.2%. White solid, mp. 50.5-52.8 oC, HPLC: 98.5%. 1H NMR (300 MHz, DMSO-d6) δ: 8.06 (dd, J = 8.7, 3.3 Hz, 1H, Ar-H), 7.93 - 7.72 (m, 2H, Ar-H), 4.08 (d, J = 2.9 Hz, 4H), 1.22 - 1.08 (m, 3H).13C NMR (75 MHz, DMSO-d6) δ: 169.42, 136.17, 132.22, 131.70, 127.42, 126.80, 60.60, 38.29, 13.97.
References:
Yu, Zutao;Chen, Zhuo;Su, Qiongli;Ye, Shiqi;Yuan, Hongbo;Kuai, Mengni;Lv, Meng;Tu, Zhijun;Yang, Xiaoping;Liu, RangRu;Hu, Gaoyun;Li, Qianbin [Bioorganic and Medicinal Chemistry,2019,vol. 27,# 6,p. 944 - 954] Location in patent:supporting information

64-17-5
825 suppliers
$10.00/50g

124840-61-5
43 suppliers
$40.00/100mg

870274-21-8
11 suppliers
inquiry

321-23-3
256 suppliers
$10.00/1g

870274-21-8
11 suppliers
inquiry