ETHYL 2-(5-BROMOPYRIMIDIN-2-YL)-2-CYANOACETATE synthesis
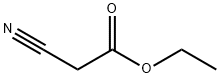
- Product Name:ETHYL 2-(5-BROMOPYRIMIDIN-2-YL)-2-CYANOACETATE
- CAS Number:65364-66-1
- Molecular formula:C9H8BrN3O2
- Molecular Weight:270.08
Yield:65364-66-1 24%
Reaction Conditions:
Stage #1: ethyl 2-cyanoacetatewith sodium hydride in tetrahydrofuran; for 0.166667 h;
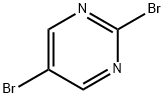
Stage #2: 2,5-dibromopyrimidine in tetrahydrofuran at 20; for 6 h;
Stage #3: with hydrogenchloride in water;
Steps:
Synthesis of ethyl (5-bromopynmιdιn-2-yl)(cvano)acetateTo a chilled stirred suspension of sodium hydride (60% in oil, 34 55 mmol) in tetrahydrofuran (20 mL) was added 1 17g of ethyl cyanoacetate (10 34 mmol) portionwise over 10 minutes After effervescence has ceased, the yellow cloudy mixture was left to warm up to room temperature whilst stirring 2 0O g of 5-bromo,2- bromo-py?midine (10 34mmol) in 3OmL of tetrahydrofuran was added And the mixture was left to stir at room temperature for six hours The resultant brown mixture was concentrated under reduced pressure to give a bright brown precipitate to which water ( 200 mL) was added After extraction with heptane ( 200 mL), the aqueous phase was acidified by the addition of HCl IM and the resultant precipitate was collected by filtration to give on drying ethyl (5 bromopy?midin 2- yl)(cyano)acetate as a b?ght brown powder (0 67g, yield = 24%) 1H NMR δ (ppm, DMSOd6) 3 5 (b, IH), 8 9 (b, IH), 8 45 (b, IH), 4 24 (t, 2H), 1 25 (t, 3H)
References:
WO2006/117356,2006,A1 Location in patent:Page/Page column 29