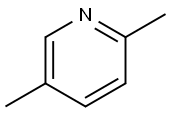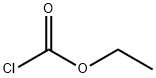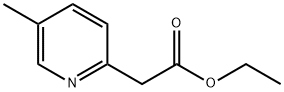
ethyl 2-(5-methylpyridin-2-yl)acetate synthesis
- Product Name:ethyl 2-(5-methylpyridin-2-yl)acetate
- CAS Number:5552-82-9
- Molecular formula:C10H13NO2
- Molecular Weight:179.22
Yield: 26%
Reaction Conditions:
Stage #1:2,5-dimethylpyridine with n-butyllithium;N,N,N,N,-tetramethylethylenediamine;N-ethyl-N,N-diisopropylamine in tetrahydrofuran;hexane at -50 - 0; for 2 h;
Stage #2:chloroformic acid ethyl ester in tetrahydrofuran;hexane at -78 - 20;
Steps:
237A Example 237A Ethyl (5-methylpyridin-2-yl)acetate
At -50° C., 179 ml (287 mmol) of a 1.6-molar solution of n-butyllithium in hexane were added dropwise to 40.2 ml (29.1 g, 287 mmol) of N,N-diisopropylethylamine and 14.0 ml (10.8 g, 92.8 mmol) of N,N,N,N-tetramethylethylenediamine in 115 ml of THF, and the mixture was stirred at -50° C. for 1 h. 15.1 ml (14.0 g, 130 mmol) of 2,5-dimethylpyridine were then added dropwise, and the mixture was stirred at 0° C. for 1 h. 12.5 ml (14.2 g, 131 mmol) of ethyl chloroformate were then added dropwise at -78° C., and the mixture was stirred at room temperature overnight.
At 0° C., first 40 ml of saturated aqueous ammonium chloride solution and then 30 ml of saturated aqueous sodium chloride solution were then added dropwise.
At room temperature, ethyl acetate was added, the phases were separated, the aqueous phase was extracted twice with ethyl acetate and the combined organic phases were washed with saturated aqueous sodium chloride solution and dried over sodium sulphate.
After filtration, the filtrate was concentrated under reduced pressure and the residue was dried under high vacuum, dissolved in cyclohexane/ethyl acetate and purified by silica gel chromatography (cyclohexane/ethyl acetate 10:1-10:5).
This gave 6.12 g (26% of theory) of the desired product.
LC-MS (Method 1A): Rt=0.83 min; MS (ESIpos): m/z=180 [M+H]+
References:
BAYER PHARMA AKTIENGESELLSCHAFT;ALLERHEILlGEN, Swen;BUCHMÜLLER, Anja;ENGEL, Karen;GERDES, Christoph;GERICKE, Kersten Matthias;GERISCH, Michael;HEITMEIER, Stefan;HILLlSCH, Alexander;KINZEL, Tom;LIENAU, Philip;RIEDL, Bernd;RÖHRIG, Susanne;SCHMIDT, Martina Victoria;STRASSBURGER, Julia;TERSTEEGEN, Adrian US2016/108027, 2016, A1 Location in patent:Paragraph 1579-1580

38203-08-6
40 suppliers
inquiry

5552-82-9
19 suppliers
$439.00/1g
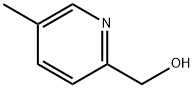
22940-71-2
68 suppliers
$45.00/50mg

5552-82-9
19 suppliers
$439.00/1g

772-71-4
7 suppliers
inquiry

5552-82-9
19 suppliers
$439.00/1g

767-01-1
40 suppliers
$34.00/100mg

5552-82-9
19 suppliers
$439.00/1g