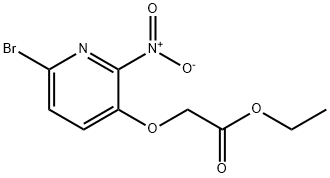
ethyl 2-(6-bromo-2-nitropyridin-3-yloxy)acetate synthesis
- Product Name:ethyl 2-(6-bromo-2-nitropyridin-3-yloxy)acetate
- CAS Number:443956-09-0
- Molecular formula:C9H9BrN2O5
- Molecular Weight:305.08
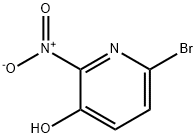
443956-08-9
149 suppliers
$11.00/250mg

105-36-2
343 suppliers
$5.00/5G

443956-09-0
12 suppliers
inquiry
Yield:443956-09-0 89%
Reaction Conditions:
with potassium carbonate in acetone; for 10 h;Heating / reflux;
Steps:
3.b
2-Bromo-5-hydroxy-6-nitropyridine (30 g, 0.14 mole) was suspended in acetone(200 ml), and potassium carbonate (39 g, 0.28 mole) was added, followed by ethyl bromoacetate (15.7 ml, 0.14 mmole). The reaction was heated at reflux for 10 hr, then was cooled to room temperature and diluted with Et2θ. The precipitate was removed by suction filtration, and the filtrate was concentrated in vacuo to afford material (38 g, 89%), which was used without further purification: MS (ES) m/z 305.0 (M + H)+.
References:
WO2006/81178,2006,A2 Location in patent:Page/Page column 22
15128-82-2
428 suppliers
$10.00/10g

443956-09-0
12 suppliers
inquiry