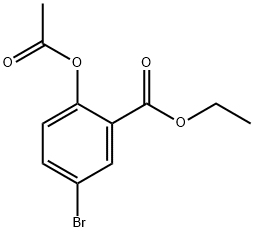
Ethyl 2-acetoxy-5-broMobenzoate synthesis
- Product Name:Ethyl 2-acetoxy-5-broMobenzoate
- CAS Number:1131622-49-5
- Molecular formula:C11H11BrO4
- Molecular Weight:287.11

37540-59-3
29 suppliers
$19.00/500mg

108-24-7
5 suppliers
$14.00/250ML

1131622-49-5
5 suppliers
inquiry
Yield:1131622-49-5 98%
Reaction Conditions:
with triethylamine;dmap at 20; for 24 h;
Steps:
7
In a 50 ml. round-bottomed flask fitted with condenser and magnetic stirrer were placed methyl 5-bromosalicylate (5.0 g, 21.64 mmol) , AC2O (2.65 g, 25.97 mmol), Et3N (2.63 g, 25.97 mmol) and catalytic amount of DMAP. The reaction mixture was stirred for 24 h at rt. The reaction mixture was poured into water and extracted with EtOAc. The organic layer was washed with water, dried and concentrated to give the acetoxy derivative (5.9 g, 98%). To a stirred solution of methyl 2-acetoxy-5-bromobenzoate (5.9 g, 21.61 mmol) in acetonitrile (80 ml_) were added 4-acetoxystyrene (3.68 g, 22.69 mmol), N1N- diisopropylethylamine (8.38 g, 64.82 mmol), biphenyl-2-yl-di-t-butyl phosphine (368 mg, 1.29 mmol) and palladium acetate (291 mg, 1.29 mmol) under N2. The reaction mixture was heated to 80 °C for 48 h and then cooled to rt. Water was added and the mixture was extracted with EtOAc. The organic layer was separated, washed with water, brine, dried and concentrated to give crude EPO
References:
WO2006/45010,2006,A2 Location in patent:Page/Page column 162-163