
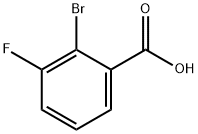
ethyl 2-broMo-3-fluorobenzoate synthesis
- Product Name:ethyl 2-broMo-3-fluorobenzoate
- CAS Number:1131040-49-7
- Molecular formula:C9H8BrFO2
- Molecular Weight:247.06

64-17-5
707 suppliers
$10.00/50g
132715-69-6
231 suppliers
$15.00/2g

1131040-49-7
19 suppliers
$60.00/100mg
Yield:1131040-49-7 93.7%
Reaction Conditions:
with thionyl chloride at 85; for 3 h;
Steps:
26.26a Step 26a: Preparation of Ethyl 3-fluoro-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzoate (Compound 0703-63 )
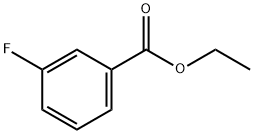
A mixture of 2-bromo-3-fluorobenzoic acid (1.0 g, 4.58 mmol, 1.0 equivalent) and thionyl chloride (546 mg, 4.58 mmol, 1.0 equivalent) in ethanol (20 mL) was stirred at 85C for 3 hours. Water and ethyl acetate were added for extraction, and the organic layer was washed with saturated brine and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate = 500/1 to 100/1) to give ethyl 2-bromo-3-fluorobenzoate (1.05 g, yield) as a colorless oil Rate: 93.7%). Under the protection of nitrogen, the ethyl 2-bromo-3-fluorobenzoate (600 mg, 2.44 mmol, 1.0 equivalent) obtained above, pinacol diboronic acid ester (1.12 g, 4.39 mmol, 1.80 equivalent), A mixture of potassium acetate (717 mg, 7.32 mmol, 3.0 equiv) and tetrakis(triphenylphosphine) palladium (141 mg, 0.122 mmol, 0.05 equiv) in toluene (30 ml) was stirred at 115Cnight. After cooling to room temperature, the mixture was filtered, and the filtrate was concentrated under reduced pressure to obtain a brown oil, 3-fluoro-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborane-2- Base) ethyl benzoate (500 mg, crude).
References:
CN112159405,2021,A Location in patent:Paragraph 0303-0304
451-02-5
140 suppliers
$15.00/25g

1131040-49-7
19 suppliers
$60.00/100mg