
ETHYL 2-CHLORO-3-NITROBENZOATE synthesis
- Product Name:ETHYL 2-CHLORO-3-NITROBENZOATE
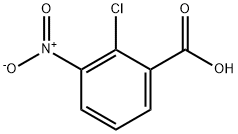
- CAS Number:3979-45-1
- Molecular formula:C9H8ClNO4
- Molecular Weight:229.62
Yield:3979-45-1 90%
Reaction Conditions:
with sulfuric acid at 15 - 30; for 24 h;Heating / reflux;
Steps:
1
Example 1. Obtaining ethyl 2-chloro-3-nitrobenzoate (IV) IV In a 250 ml capacity flask, 5 g (1 equivalent) of 2- chloro-3-nitrobenzoic acid is dissolved in 50 ml of ethanol. 3.5 ml of sulphuric acid is then added to the solution, keeping the temperature of the reaction between15 and 30°C.The mixture is heated at reflux for 24 hours, following which the solvent is evaporated at reduced pressure and the residue is poured onto 120 ml of cold water. The mixture is extracted with toluene (3 x 50 ml) and the organic phase obtained is washed with 100 ml of water and with 100 ml of an aqueous solution of potassium carbonate. The organic phase is dried with MgSO4, filtered and concentrated to dryness at reduced pressure. The resulting product, a pale yellow oil (5.1 g, 90%), is used without further purification in the following reaction.
References:
WO2006/134078,2006,A1 Location in patent:Page/Page column 10
3970-35-2
255 suppliers
$6.00/250mg

3979-45-1
15 suppliers
inquiry
