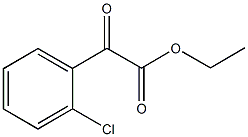
ETHYL 2-CHLOROBENZOYLFORMATE synthesis
- Product Name:ETHYL 2-CHLOROBENZOYLFORMATE
- CAS Number:62123-75-5
- Molecular formula:C10H9ClO3
- Molecular Weight:212.63
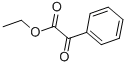
1603-79-8
202 suppliers
$7.00/5g

62123-75-5
32 suppliers
$160.41/0.5g
Yield:62123-75-5 63%
Reaction Conditions:
with 2-chlorocyclopentane-1,3-dione;palladium diacetate;(2,2,2-trifluoroacetyl)oxysilver;glacial acetic acid;3,5-ditrifluoromethylaniline;trifluoroacetic acid at 100; for 24 h;Sealed tube;
Steps:
General Procedure II
General procedure: A sealed tube with magnetic stir bar was charged with NCS (40 mg, 0.3 mmol), Pd(OAc)2 (0.02 mmol) in air, followed by HFIP:AcOH (2 mL, 9:1), α-keto esters 1 (0.2 mmol), T5 (9.1 mg, 0.04 mmol) and TFA (136.8 mg, 1.2 mmol). The reaction mixture was stirred at 100 for 24 hours. Upon completion, the reaction mixture was quenched by sat. NaHCO3 (aq) (10 mL), and extracted with CH2Cl2 for 3 times. The combined organic layers were washed with water (15 mL), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude residue was purified by column chromatography on silica gel using petroleum ether/EtOAc as eluent to afford the desired products.
References:
Wang, Jian;Wu, Yongdi;Xu, Wengang;Lu, Xuelian;Wang, Yunfang;Liu, Guangyuan;Sun, Bing;Zhou, Yirong;Zhang, Fang-Lin [Tetrahedron,2022,vol. 123,art. no. 132980] Location in patent:supporting information

3900-89-8
378 suppliers
$6.00/1g

623-49-4
233 suppliers
$10.60/1gm:

62123-75-5
32 suppliers
$160.41/0.5g

64-17-5
706 suppliers
$10.00/50g

40018-25-5
195 suppliers
$9.00/1g

62123-75-5
32 suppliers
$160.41/0.5g

64-17-5
706 suppliers
$10.00/50g
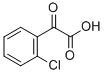
26118-14-9
34 suppliers
$45.00/10mg

62123-75-5
32 suppliers
$160.41/0.5g
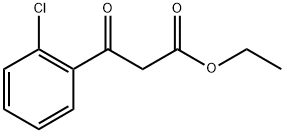
19112-35-7
88 suppliers
$16.00/1g

62123-75-5
32 suppliers
$160.41/0.5g