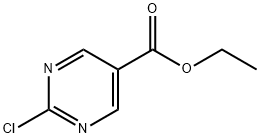
Ethyl 2-chloropyrimidine-5-carboxylate synthesis
- Product Name:Ethyl 2-chloropyrimidine-5-carboxylate
- CAS Number:89793-12-4
- Molecular formula:C7H7ClN2O2
- Molecular Weight:186.6
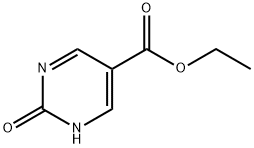
95928-49-7
93 suppliers
$45.00/50mg

89793-12-4
233 suppliers
$6.00/100mg
Yield:89793-12-4 52%
Reaction Conditions:
with N,N-dimethyl-aniline;trichlorophosphate for 2 h;Reflux;
Steps:
1.d Step d: Ethyl 2-chloropyrimidine-5-carboxylate (Compound R-2-1)
Step d:
Ethyl 2-chloropyrimidine-5-carboxylate (Compound R-2-1)
A mixture of compound 204 (38.0 g, 153 mmol) and phosphoryl trichloride (300 mL) and N,N-dimethylaniline (3 mL) was heated at reflux for 2 h, cooled to room temperature and concentrated.
The residue was quenched carefully with ice-water, adjusted pH to 7-8 with sodium carbonate and extracted with EtOAc.
The combined organics were washed with ice-water and brine, dried over Na2SO4, evaporated, and purified by column chromatography (eluted with EtOAc/Hexanes, 10%) to afford compound R-2-1 (15 g, 52%) as a white solid. LCMS: 187 [M+1]+. 1H NMR (400 MHz, CDCl3): δ 1.36 (t, J=7.5 Hz, 3H), 4.39 (q, J=7.5 Hz, 2H), 9.08 (s, 2H).
References:
Curis, Inc.;Cai, Xiong;Zhai, Haixiao;Lai, Chengjung;Qian, Changgeng;Bao, Rudi US9249156, 2016, B2 Location in patent:Page/Page column 34

95928-49-7
93 suppliers
$45.00/50mg

89793-12-4
233 suppliers
$6.00/100mg

763-69-9
313 suppliers
$14.00/25mL

89793-12-4
233 suppliers
$6.00/100mg
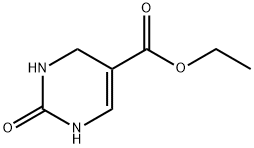
33458-27-4
27 suppliers
$152.00/500mg

89793-12-4
233 suppliers
$6.00/100mg
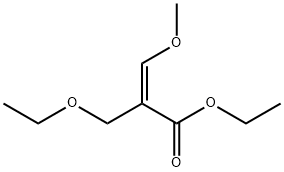
879008-23-8
0 suppliers
inquiry

89793-12-4
233 suppliers
$6.00/100mg