
ethyl 2-(chlorosulfonyl)cyclohex-1-enecarboxylate synthesis
- Product Name:ethyl 2-(chlorosulfonyl)cyclohex-1-enecarboxylate
- CAS Number:243984-26-1
- Molecular formula:C9H13ClO4S
- Molecular Weight:252.72
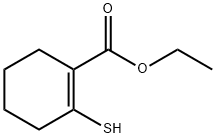
54928-91-5
10 suppliers
inquiry

243984-26-1
5 suppliers
inquiry
Yield:243984-26-1 82.5%
Reaction Conditions:
with water;chlorine in acetic acid at 15; for 0.833333 h;
Steps:
35 General procedure for preparation of compound 10:
Chlorine (10 g) was bubbled into a solution of compound 9 (25 g, 134.2 mmol, 1 eq) in AcOH (360 mL) H2O (40 mL) at 15 °C for 20 min. The reaction mixture was then stirred for 30 min. After excess Cl2 was purged by N2, TLC (Petroleum ether: Ethyl acetate = 10:1 Rf = 0.4) indicated compound 9 was consumed completely. The residue was diluted with NH4Cl (500 mL *5) and extracted with ethyl acetate (500 ml). The reaction mixture was poured into separatory funnel and separated. The combined organic layers were washed with brine (500 mL), dried over Na2SO4, filtered and concentrated under reduced pressure to give a residue. Compound 10 (28 g, 110 mmol, yield: 82.5%) was obtained as a yellow oil.1H NMR (400 MHz, CHLOROFORM-d): δ ppm 4.19 - 4.31 (m, 2 H) 2.52 - 2.61 (m, 2 H) 2.44 - 2.52 (m, 2 H) 1.72 - 1.82 (m, 2 H) 1.62 - 1.72 (m, 2 H) 1.21 - 1.33 (m, 3 H).
References:
WO2021/102052,2021,A1 Location in patent:Paragraph 0914; 0915

243984-25-0
4 suppliers
inquiry

243984-26-1
5 suppliers
inquiry
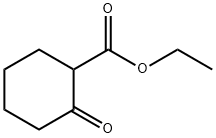
1655-07-8
311 suppliers
$6.00/5g

243984-26-1
5 suppliers
inquiry

122135-83-5
51 suppliers
$65.00/100mg

243984-26-1
5 suppliers
inquiry